Input interpretation

alkali metals | atomic volume
Summary

median | 4.5×10^7 pm^3 highest | 7.4×10^7 pm^3 (cesium) lowest | 1.28×10^7 pm^3 (lithium) distribution | | (based on 5 values; 1 unavailable)
Entity with missing value
francium
Ranked values
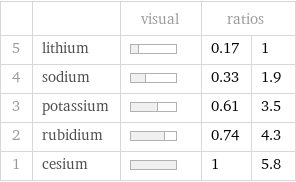
| | visual | ratios | 5 | lithium | | 0.17 | 1 4 | sodium | | 0.33 | 1.9 3 | potassium | | 0.61 | 3.5 2 | rubidium | | 0.74 | 4.3 1 | cesium | | 1 | 5.8
Plot
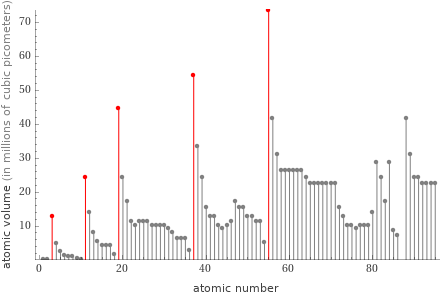
Plot
Periodic table location
Periodic table location
Atomic radii properties
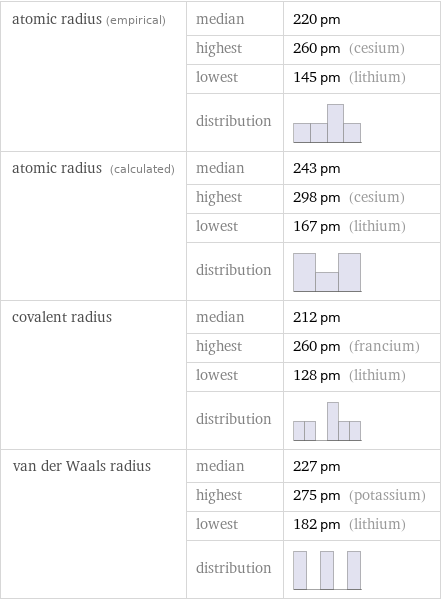
atomic radius (empirical) | median | 220 pm | highest | 260 pm (cesium) | lowest | 145 pm (lithium) | distribution | atomic radius (calculated) | median | 243 pm | highest | 298 pm (cesium) | lowest | 167 pm (lithium) | distribution | covalent radius | median | 212 pm | highest | 260 pm (francium) | lowest | 128 pm (lithium) | distribution | van der Waals radius | median | 227 pm | highest | 275 pm (potassium) | lowest | 182 pm (lithium) | distribution |
Units

Atomic volume rankings
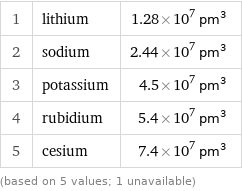
1 | lithium | 1.28×10^7 pm^3 2 | sodium | 2.44×10^7 pm^3 3 | potassium | 4.5×10^7 pm^3 4 | rubidium | 5.4×10^7 pm^3 5 | cesium | 7.4×10^7 pm^3 (based on 5 values; 1 unavailable)
Unit conversions for median atomic volume 4.5×10^7 pm^3
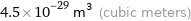
4.5×10^-29 m^3 (cubic meters)
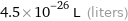
4.5×10^-26 L (liters)
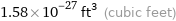
1.58×10^-27 ft^3 (cubic feet)
Corresponding quantities

Radius r of a sphere from V = 4πr^3/3: | 220 pm (picometers) | 8.7×10^-9 inches

Edge length a of a cube from V = a^3: | 355 pm (picometers) | 1.4×10^-8 inches