Input interpretation
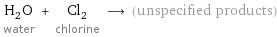
H_2O water + Cl_2 chlorine ⟶ (unspecified products)
Sample reactions
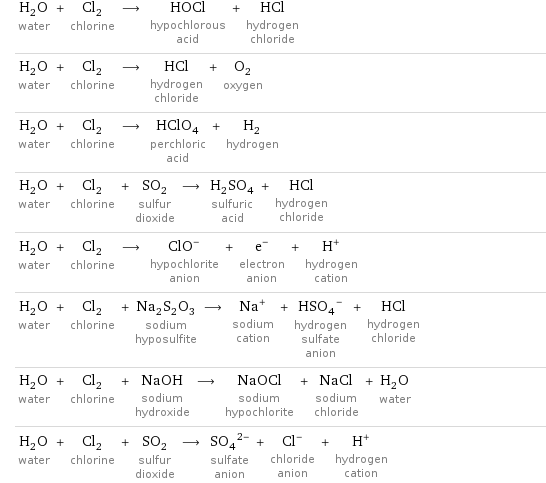
H_2O water + Cl_2 chlorine ⟶ HOCl hypochlorous acid + HCl hydrogen chloride H_2O water + Cl_2 chlorine ⟶ HCl hydrogen chloride + O_2 oxygen H_2O water + Cl_2 chlorine ⟶ HClO_4 perchloric acid + H_2 hydrogen H_2O water + Cl_2 chlorine + SO_2 sulfur dioxide ⟶ H_2SO_4 sulfuric acid + HCl hydrogen chloride H_2O water + Cl_2 chlorine ⟶ (ClO)^- hypochlorite anion + e^- electron anion + H^+ hydrogen cation H_2O water + Cl_2 chlorine + Na_2S_2O_3 sodium hyposulfite ⟶ Na^+ sodium cation + (HSO_4)^- hydrogen sulfate anion + HCl hydrogen chloride H_2O water + Cl_2 chlorine + NaOH sodium hydroxide ⟶ NaOCl sodium hypochlorite + NaCl sodium chloride + H_2O water H_2O water + Cl_2 chlorine + SO_2 sulfur dioxide ⟶ (SO_4)^(2-) sulfate anion + Cl^- chloride anion + H^+ hydrogen cation
Chemical names and formulas
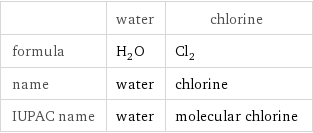
| water | chlorine formula | H_2O | Cl_2 name | water | chlorine IUPAC name | water | molecular chlorine
Substance properties
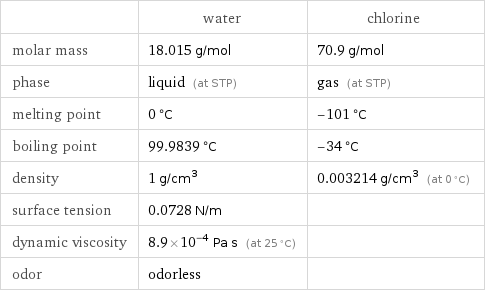
| water | chlorine molar mass | 18.015 g/mol | 70.9 g/mol phase | liquid (at STP) | gas (at STP) melting point | 0 °C | -101 °C boiling point | 99.9839 °C | -34 °C density | 1 g/cm^3 | 0.003214 g/cm^3 (at 0 °C) surface tension | 0.0728 N/m | dynamic viscosity | 8.9×10^-4 Pa s (at 25 °C) | odor | odorless |
Units
