Input interpretation
potassium hexachloroiridate(III) | magnesium antimonide
Chemical names and formulas
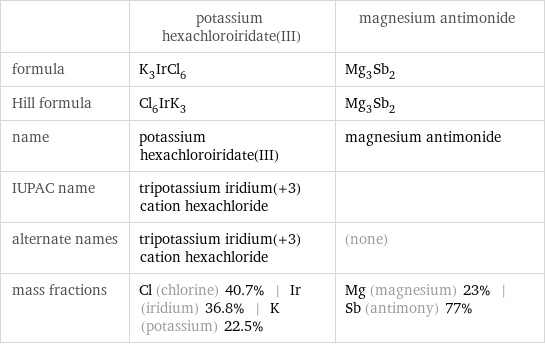
| potassium hexachloroiridate(III) | magnesium antimonide formula | K_3IrCl_6 | Mg_3Sb_2 Hill formula | Cl_6IrK_3 | Mg_3Sb_2 name | potassium hexachloroiridate(III) | magnesium antimonide IUPAC name | tripotassium iridium(+3) cation hexachloride | alternate names | tripotassium iridium(+3) cation hexachloride | (none) mass fractions | Cl (chlorine) 40.7% | Ir (iridium) 36.8% | K (potassium) 22.5% | Mg (magnesium) 23% | Sb (antimony) 77%
Structure diagrams
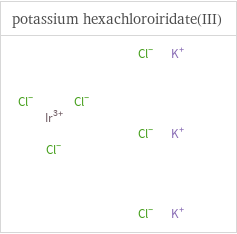
Structure diagrams
Basic properties

| potassium hexachloroiridate(III) | magnesium antimonide molar mass | 522.2 g/mol | 316.4 g/mol melting point | | 1245 °C density | | 3.99 g/cm^3
Units
