Input interpretation
tin(II) 2-ethylhexanoate | silver(I) cyanate
Chemical names and formulas
![| tin(II) 2-ethylhexanoate | silver(I) cyanate formula | [CH_3(CH_2)_3CH(C_2H_5)CO_2]_2Sn | AgOCN Hill formula | C_16H_30O_4Sn | CHAgNO name | tin(II) 2-ethylhexanoate | silver(I) cyanate IUPAC name | stannous 2-ethylhexanoate | cyanic acid; silver alternate names | 2-ethylhexanoic acid tin(II) salt | stannous 2-ethylhexanoate | stannous octoate | cyanic acid; silver | cyanic acid, silver(1+) salt | silver cyanate mass fractions | C (carbon) 47.4% | H (hydrogen) 7.46% | O (oxygen) 15.8% | Sn (tin) 29.3% | Ag (silver) 71.5% | C (carbon) 7.96% | H (hydrogen) 0.668% | N (nitrogen) 9.28% | O (oxygen) 10.6%](../image_source/bab2ea3827ea925e8d1fe24aba8a992d.png)
| tin(II) 2-ethylhexanoate | silver(I) cyanate formula | [CH_3(CH_2)_3CH(C_2H_5)CO_2]_2Sn | AgOCN Hill formula | C_16H_30O_4Sn | CHAgNO name | tin(II) 2-ethylhexanoate | silver(I) cyanate IUPAC name | stannous 2-ethylhexanoate | cyanic acid; silver alternate names | 2-ethylhexanoic acid tin(II) salt | stannous 2-ethylhexanoate | stannous octoate | cyanic acid; silver | cyanic acid, silver(1+) salt | silver cyanate mass fractions | C (carbon) 47.4% | H (hydrogen) 7.46% | O (oxygen) 15.8% | Sn (tin) 29.3% | Ag (silver) 71.5% | C (carbon) 7.96% | H (hydrogen) 0.668% | N (nitrogen) 9.28% | O (oxygen) 10.6%
Structure diagrams
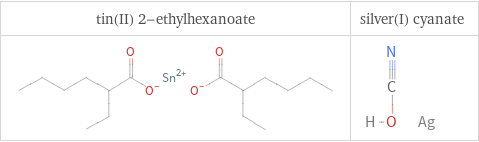
Structure diagrams
Basic properties

| tin(II) 2-ethylhexanoate | silver(I) cyanate molar mass | 405.12 g/mol | 150.89 g/mol density | 1.251 g/cm^3 | 4 g/cm^3
Units
