Input interpretation

gallium(III) fluoride trihydrate | element types
Result
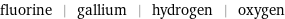
fluorine | gallium | hydrogen | oxygen
Periodic table location
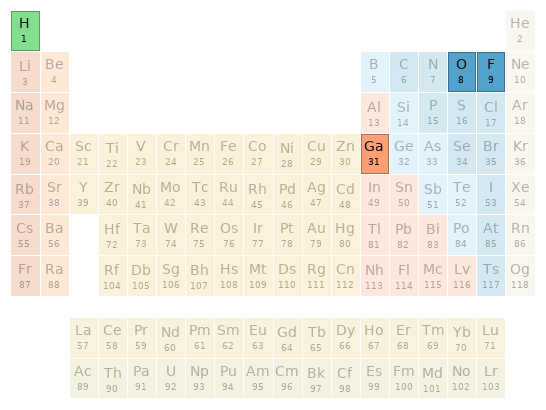
Periodic table location
Images
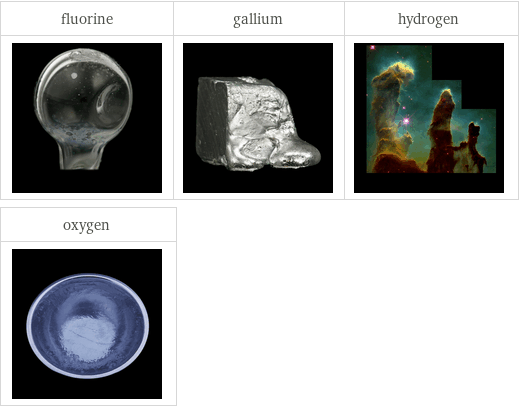
Images
Basic elemental properties
![| atomic symbol | atomic number | short electronic configuration fluorine | F | 9 | [He]2s^22p^5 gallium | Ga | 31 | [Ar]4s^23d^104p^1 hydrogen | H | 1 | 1s^1 oxygen | O | 8 | [He]2s^22p^4 | Aufbau diagram | block | group fluorine | 2p 2s | p | 17 gallium | 4p 3d 4s | p | 13 hydrogen | 1s | s | 1 oxygen | 2p 2s | p | 16 | period | atomic mass | half-life fluorine | 2 | 18.998403163 u | (stable) gallium | 4 | 69.723 u | (stable) hydrogen | 1 | 1.008 u | (stable) oxygen | 2 | 15.999 u | (stable)](../image_source/b81414c5e4b6f3995b757a56c1228303.png)
| atomic symbol | atomic number | short electronic configuration fluorine | F | 9 | [He]2s^22p^5 gallium | Ga | 31 | [Ar]4s^23d^104p^1 hydrogen | H | 1 | 1s^1 oxygen | O | 8 | [He]2s^22p^4 | Aufbau diagram | block | group fluorine | 2p 2s | p | 17 gallium | 4p 3d 4s | p | 13 hydrogen | 1s | s | 1 oxygen | 2p 2s | p | 16 | period | atomic mass | half-life fluorine | 2 | 18.998403163 u | (stable) gallium | 4 | 69.723 u | (stable) hydrogen | 1 | 1.008 u | (stable) oxygen | 2 | 15.999 u | (stable)
Thermodynamic properties
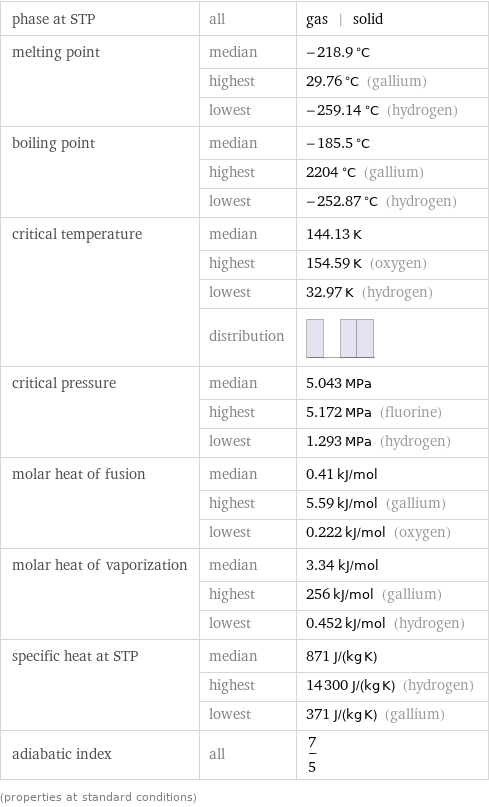
phase at STP | all | gas | solid melting point | median | -218.9 °C | highest | 29.76 °C (gallium) | lowest | -259.14 °C (hydrogen) boiling point | median | -185.5 °C | highest | 2204 °C (gallium) | lowest | -252.87 °C (hydrogen) critical temperature | median | 144.13 K | highest | 154.59 K (oxygen) | lowest | 32.97 K (hydrogen) | distribution | critical pressure | median | 5.043 MPa | highest | 5.172 MPa (fluorine) | lowest | 1.293 MPa (hydrogen) molar heat of fusion | median | 0.41 kJ/mol | highest | 5.59 kJ/mol (gallium) | lowest | 0.222 kJ/mol (oxygen) molar heat of vaporization | median | 3.34 kJ/mol | highest | 256 kJ/mol (gallium) | lowest | 0.452 kJ/mol (hydrogen) specific heat at STP | median | 871 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 371 J/(kg K) (gallium) adiabatic index | all | 7/5 (properties at standard conditions)
Material properties
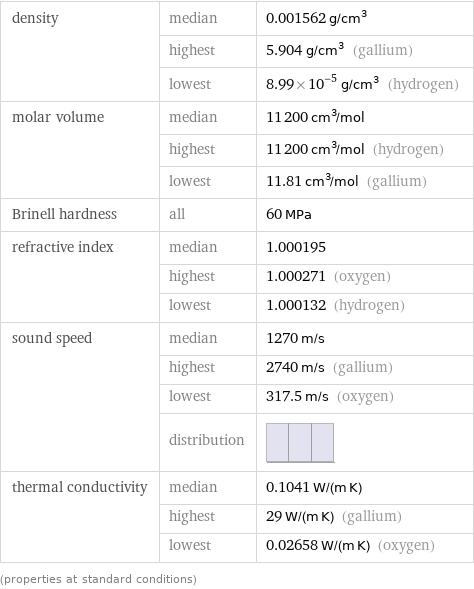
density | median | 0.001562 g/cm^3 | highest | 5.904 g/cm^3 (gallium) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) molar volume | median | 11200 cm^3/mol | highest | 11200 cm^3/mol (hydrogen) | lowest | 11.81 cm^3/mol (gallium) Brinell hardness | all | 60 MPa refractive index | median | 1.000195 | highest | 1.000271 (oxygen) | lowest | 1.000132 (hydrogen) sound speed | median | 1270 m/s | highest | 2740 m/s (gallium) | lowest | 317.5 m/s (oxygen) | distribution | thermal conductivity | median | 0.1041 W/(m K) | highest | 29 W/(m K) (gallium) | lowest | 0.02658 W/(m K) (oxygen) (properties at standard conditions)
Reactivity
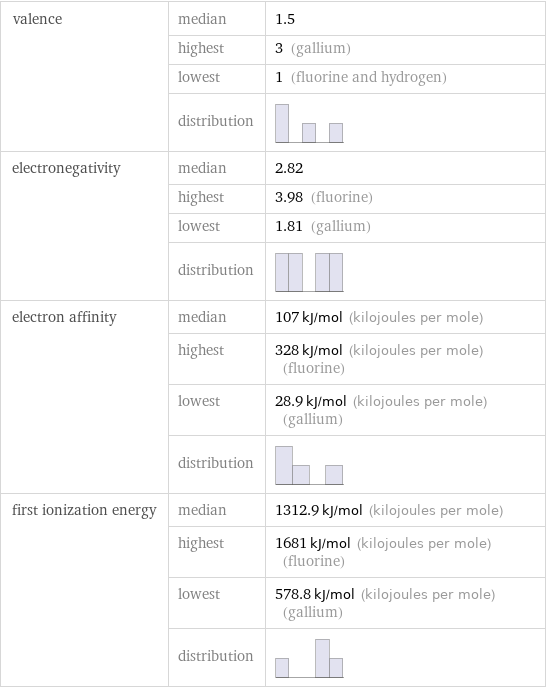
valence | median | 1.5 | highest | 3 (gallium) | lowest | 1 (fluorine and hydrogen) | distribution | electronegativity | median | 2.82 | highest | 3.98 (fluorine) | lowest | 1.81 (gallium) | distribution | electron affinity | median | 107 kJ/mol (kilojoules per mole) | highest | 328 kJ/mol (kilojoules per mole) (fluorine) | lowest | 28.9 kJ/mol (kilojoules per mole) (gallium) | distribution | first ionization energy | median | 1312.9 kJ/mol (kilojoules per mole) | highest | 1681 kJ/mol (kilojoules per mole) (fluorine) | lowest | 578.8 kJ/mol (kilojoules per mole) (gallium) | distribution |
Atomic properties
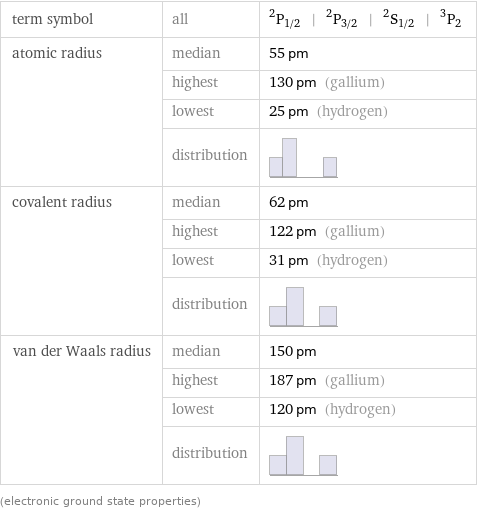
term symbol | all | ^2P_(1/2) | ^2P_(3/2) | ^2S_(1/2) | ^3P_2 atomic radius | median | 55 pm | highest | 130 pm (gallium) | lowest | 25 pm (hydrogen) | distribution | covalent radius | median | 62 pm | highest | 122 pm (gallium) | lowest | 31 pm (hydrogen) | distribution | van der Waals radius | median | 150 pm | highest | 187 pm (gallium) | lowest | 120 pm (hydrogen) | distribution | (electronic ground state properties)
Abundances
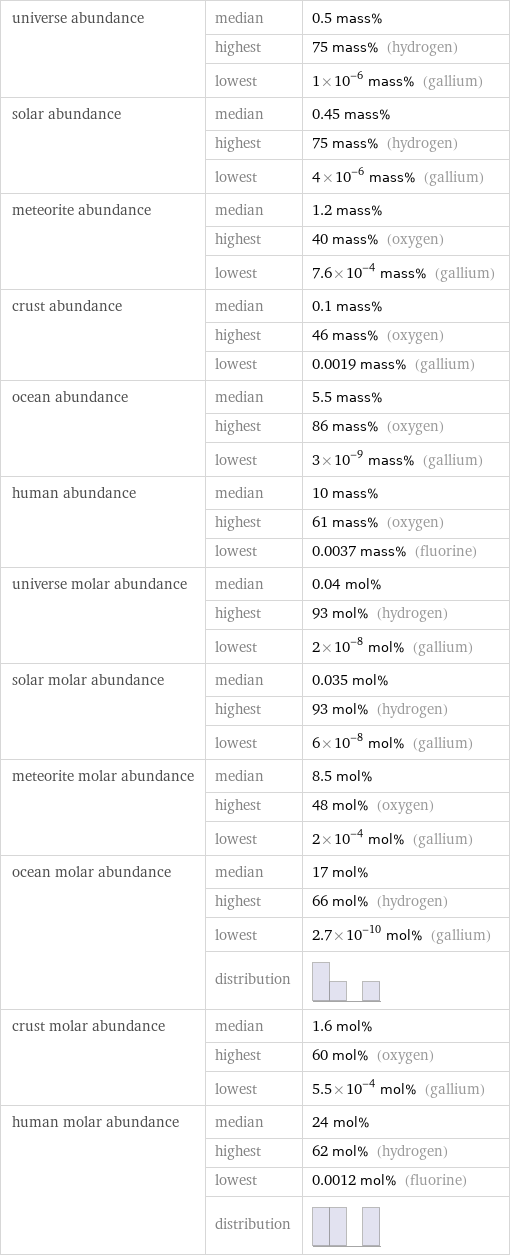
universe abundance | median | 0.5 mass% | highest | 75 mass% (hydrogen) | lowest | 1×10^-6 mass% (gallium) solar abundance | median | 0.45 mass% | highest | 75 mass% (hydrogen) | lowest | 4×10^-6 mass% (gallium) meteorite abundance | median | 1.2 mass% | highest | 40 mass% (oxygen) | lowest | 7.6×10^-4 mass% (gallium) crust abundance | median | 0.1 mass% | highest | 46 mass% (oxygen) | lowest | 0.0019 mass% (gallium) ocean abundance | median | 5.5 mass% | highest | 86 mass% (oxygen) | lowest | 3×10^-9 mass% (gallium) human abundance | median | 10 mass% | highest | 61 mass% (oxygen) | lowest | 0.0037 mass% (fluorine) universe molar abundance | median | 0.04 mol% | highest | 93 mol% (hydrogen) | lowest | 2×10^-8 mol% (gallium) solar molar abundance | median | 0.035 mol% | highest | 93 mol% (hydrogen) | lowest | 6×10^-8 mol% (gallium) meteorite molar abundance | median | 8.5 mol% | highest | 48 mol% (oxygen) | lowest | 2×10^-4 mol% (gallium) ocean molar abundance | median | 17 mol% | highest | 66 mol% (hydrogen) | lowest | 2.7×10^-10 mol% (gallium) | distribution | crust molar abundance | median | 1.6 mol% | highest | 60 mol% (oxygen) | lowest | 5.5×10^-4 mol% (gallium) human molar abundance | median | 24 mol% | highest | 62 mol% (hydrogen) | lowest | 0.0012 mol% (fluorine) | distribution |