Input interpretation
rhenium oxide | tetrabasic lead(II) sulfate
Chemical names and formulas
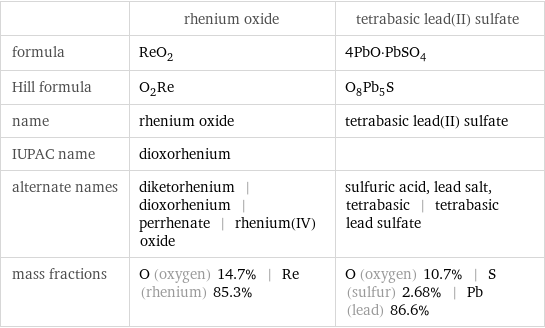
| rhenium oxide | tetrabasic lead(II) sulfate formula | ReO_2 | 4PbO·PbSO_4 Hill formula | O_2Re | O_8Pb_5S name | rhenium oxide | tetrabasic lead(II) sulfate IUPAC name | dioxorhenium | alternate names | diketorhenium | dioxorhenium | perrhenate | rhenium(IV) oxide | sulfuric acid, lead salt, tetrabasic | tetrabasic lead sulfate mass fractions | O (oxygen) 14.7% | Re (rhenium) 85.3% | O (oxygen) 10.7% | S (sulfur) 2.68% | Pb (lead) 86.6%
Structure diagrams
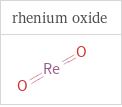
Structure diagrams
Basic properties
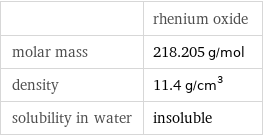
| rhenium oxide molar mass | 218.205 g/mol density | 11.4 g/cm^3 solubility in water | insoluble
Units
