Input interpretation
500 mg of silver nitrate
Basic properties for 500 mg

mass | 0.5 grams 5×10^-4 kg (kilograms) molar amount | 2.94 mmol (millimoles) 0.00294 mol (moles) equivalents | 0.0029 eq (equivalents) of silver(I) cation 0.0029 eq (equivalents) of nitrate anion (at STP)
Thermodynamic properties for 500 mg
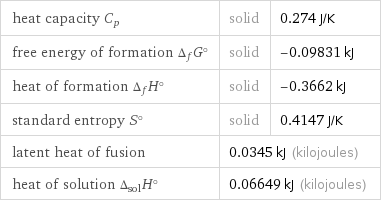
heat capacity C_p | solid | 0.274 J/K free energy of formation Δ_fG° | solid | -0.09831 kJ heat of formation Δ_fH° | solid | -0.3662 kJ standard entropy S° | solid | 0.4147 J/K latent heat of fusion | 0.0345 kJ (kilojoules) | heat of solution Δ_solH° | 0.06649 kJ (kilojoules) |
Units

Phase change energies for 500 mg from 25 °C

energy required to heat to melting point | 0.0512 kJ (kilojoules) energy required to convert to liquid | 0.0345 kJ (kilojoules) energy required to heat to melting point and convert to liquid | 0.0857 kJ (kilojoules)
Mass composition for 500 mg
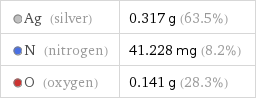
Ag (silver) | 0.317 g (63.5%) N (nitrogen) | 41.228 mg (8.2%) O (oxygen) | 0.141 g (28.3%)
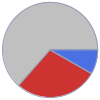
Mass composition for 500 mg
Structure diagram
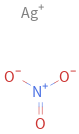
Structure diagram
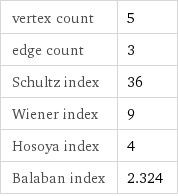
vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Chemical names and formulas
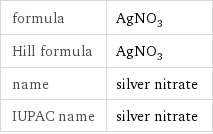
formula | AgNO_3 Hill formula | AgNO_3 name | silver nitrate IUPAC name | silver nitrate
Substance properties
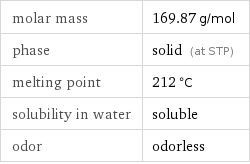
molar mass | 169.87 g/mol phase | solid (at STP) melting point | 212 °C solubility in water | soluble odor | odorless
Units
