Input interpretation

naturally occurring elements | molar heat of fusion
Summary
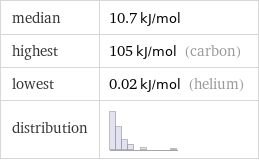
median | 10.7 kJ/mol highest | 105 kJ/mol (carbon) lowest | 0.02 kJ/mol (helium) distribution |
Units

Distribution plots
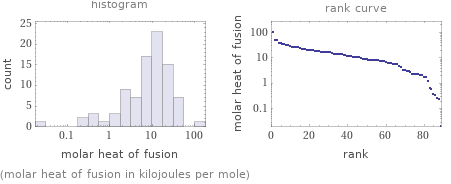
(molar heat of fusion in kilojoules per mole)
Molar heat of fusion rankings
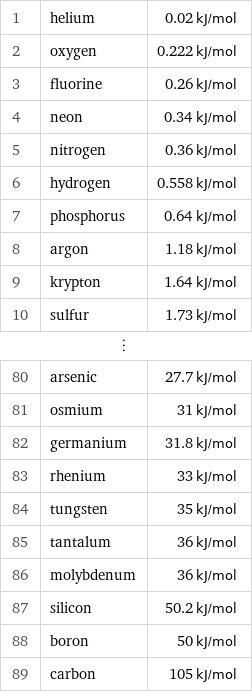
1 | helium | 0.02 kJ/mol 2 | oxygen | 0.222 kJ/mol 3 | fluorine | 0.26 kJ/mol 4 | neon | 0.34 kJ/mol 5 | nitrogen | 0.36 kJ/mol 6 | hydrogen | 0.558 kJ/mol 7 | phosphorus | 0.64 kJ/mol 8 | argon | 1.18 kJ/mol 9 | krypton | 1.64 kJ/mol 10 | sulfur | 1.73 kJ/mol ⋮ | | 80 | arsenic | 27.7 kJ/mol 81 | osmium | 31 kJ/mol 82 | germanium | 31.8 kJ/mol 83 | rhenium | 33 kJ/mol 84 | tungsten | 35 kJ/mol 85 | tantalum | 36 kJ/mol 86 | molybdenum | 36 kJ/mol 87 | silicon | 50.2 kJ/mol 88 | boron | 50 kJ/mol 89 | carbon | 105 kJ/mol
Unit conversions for median molar heat of fusion 10.7 kJ/mol
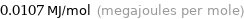
0.0107 MJ/mol (megajoules per mole)
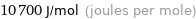
10700 J/mol (joules per mole)

2.56 kcal/mol (thermochemical kilocalories per mole)
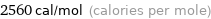
2560 cal/mol (calories per mole)

0.111 eV (molar electronvolts)