Input interpretation

(sodium bromate gallium)/gallium
Result
sodium bromate
Chemical names and formulas

formula | NaBrO_3 Hill formula | BrNaO_3 name | sodium bromate alternate names | DOT | dyetone mass fractions | Br (bromine) 53% | Na (sodium) 15.2% | O (oxygen) 31.8%
Structure diagram
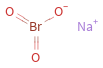
Structure diagram

vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties

molar mass | 150.89 g/mol phase | solid (at STP) melting point | 381 °C boiling point | 1390 °C density | 3.339 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)

density | 3.339 g/cm^3
Units

Thermodynamic properties
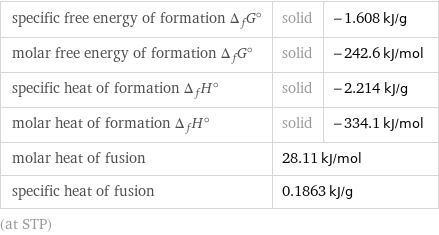
specific free energy of formation Δ_fG° | solid | -1.608 kJ/g molar free energy of formation Δ_fG° | solid | -242.6 kJ/mol specific heat of formation Δ_fH° | solid | -2.214 kJ/g molar heat of formation Δ_fH° | solid | -334.1 kJ/mol molar heat of fusion | 28.11 kJ/mol | specific heat of fusion | 0.1863 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7789-38-0 PubChem CID number | 23668195 PubChem SID number | 24853461 SMILES identifier | [O-]Br(=O)=O.[Na+] InChI identifier | InChI=1/BrHO3.Na/c2-1(3)4;/h(H, 2, 3, 4);/q;+1/p-1/fBrO3.Na/q-1;m RTECS number | EF8750000 MDL number | MFCD00003476](../image_source/1da576db4058fb1b8da29fc0325923f9.png)
CAS number | 7789-38-0 PubChem CID number | 23668195 PubChem SID number | 24853461 SMILES identifier | [O-]Br(=O)=O.[Na+] InChI identifier | InChI=1/BrHO3.Na/c2-1(3)4;/h(H, 2, 3, 4);/q;+1/p-1/fBrO3.Na/q-1;m RTECS number | EF8750000 MDL number | MFCD00003476
NFPA label

NFPA label

NFPA hazards | oxidizing agent
Safety properties

flash point | 381 °C

DOT hazard class | 5.1 DOT numbers | 1494
Toxicity properties

odor | odorless

RTECS classes | tumorigen | human data
Ion equivalents

Na^+ (sodium cation) | 1 (BrO_3)^- (bromate anion) | 1