Input interpretation

manganese dioxide | potassium fluoride dihydrate
Chemical names and formulas
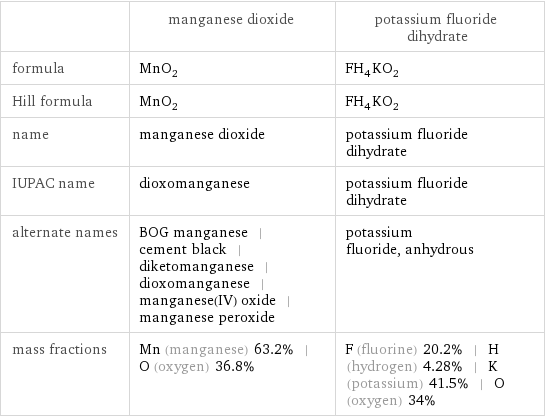
| manganese dioxide | potassium fluoride dihydrate formula | MnO_2 | FH_4KO_2 Hill formula | MnO_2 | FH_4KO_2 name | manganese dioxide | potassium fluoride dihydrate IUPAC name | dioxomanganese | potassium fluoride dihydrate alternate names | BOG manganese | cement black | diketomanganese | dioxomanganese | manganese(IV) oxide | manganese peroxide | potassium fluoride, anhydrous mass fractions | Mn (manganese) 63.2% | O (oxygen) 36.8% | F (fluorine) 20.2% | H (hydrogen) 4.28% | K (potassium) 41.5% | O (oxygen) 34%
Structure diagrams
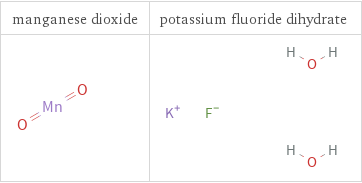
Structure diagrams
Basic properties
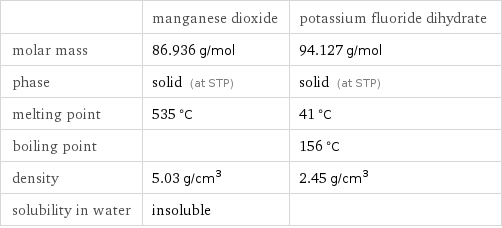
| manganese dioxide | potassium fluoride dihydrate molar mass | 86.936 g/mol | 94.127 g/mol phase | solid (at STP) | solid (at STP) melting point | 535 °C | 41 °C boiling point | | 156 °C density | 5.03 g/cm^3 | 2.45 g/cm^3 solubility in water | insoluble |
Units
