Input interpretation
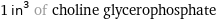
1 in^3 of choline glycerophosphate
Basic properties for 1 in^3
mass | 13 grams 0.013 kg (kilograms) 0.457 oz (ounces) molar amount | 50.4 mmol (millimoles) 0.0504 mol (moles) volume | 16.4 mL (milliliters) 0.0164 L (liters) 16.4 cm^3 (cubic centimeters) 1.64×10^-5 m^3 (cubic meters) 0.554 fl oz (fluid ounces) 3.32 tsp (teaspoons) 1.11 tbsp (tablespoons) 0.0173 quarts equivalents | 0.0504 eq (equivalents) (at STP)
Corresponding quantities
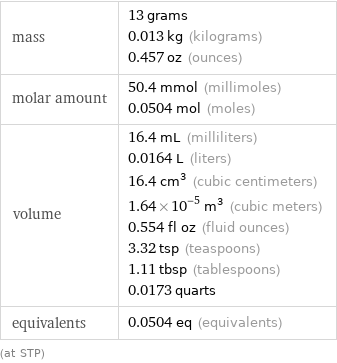
sphere radius | 1.576 cm (centimeters) side of a cube | 2.54 cm (centimeters)
Mass composition for 1 in^3
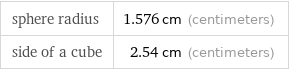
C (carbon) | 4.840 g (37.4%) H (hydrogen) | 1.020 g (7.8%) N (nitrogen) | 0.706 g (5.4%) O (oxygen) | 4.840 g (37.3%) P (phosphorus) | 1.560 g (12.0%)
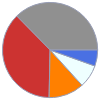
Mass composition for 1 in^3
Lewis structure
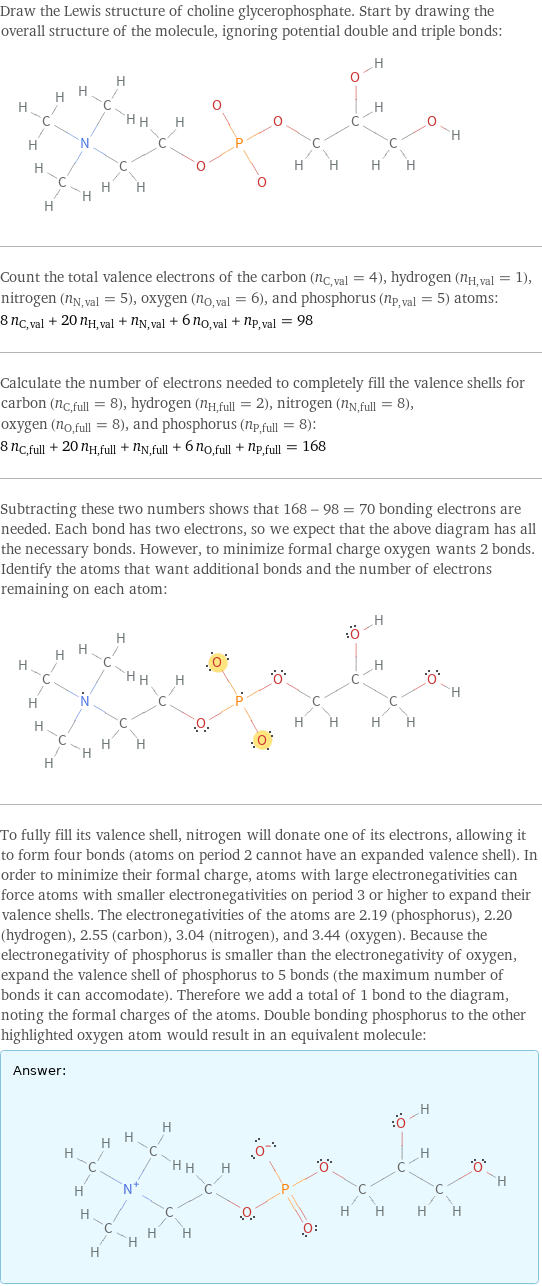
Draw the Lewis structure of choline glycerophosphate. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), nitrogen (n_N, val = 5), oxygen (n_O, val = 6), and phosphorus (n_P, val = 5) atoms: 8 n_C, val + 20 n_H, val + n_N, val + 6 n_O, val + n_P, val = 98 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), nitrogen (n_N, full = 8), oxygen (n_O, full = 8), and phosphorus (n_P, full = 8): 8 n_C, full + 20 n_H, full + n_N, full + 6 n_O, full + n_P, full = 168 Subtracting these two numbers shows that 168 - 98 = 70 bonding electrons are needed. Each bond has two electrons, so we expect that the above diagram has all the necessary bonds. However, to minimize formal charge oxygen wants 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: To fully fill its valence shell, nitrogen will donate one of its electrons, allowing it to form four bonds (atoms on period 2 cannot have an expanded valence shell). In order to minimize their formal charge, atoms with large electronegativities can force atoms with smaller electronegativities on period 3 or higher to expand their valence shells. The electronegativities of the atoms are 2.19 (phosphorus), 2.20 (hydrogen), 2.55 (carbon), 3.04 (nitrogen), and 3.44 (oxygen). Because the electronegativity of phosphorus is smaller than the electronegativity of oxygen, expand the valence shell of phosphorus to 5 bonds (the maximum number of bonds it can accomodate). Therefore we add a total of 1 bond to the diagram, noting the formal charges of the atoms. Double bonding phosphorus to the other highlighted oxygen atom would result in an equivalent molecule: Answer: | |
Chemical names and formulas
![formula | C_8H_20NO_6P Hill formula | C_8H_20NO_6P name | choline glycerophosphate IUPAC name | 2-[[(2S)-2, 3-dihydroxypropoxy]-oxido-phosphoryl]oxyethyl-trimethyl-azanium](../image_source/d253f31ccba29a7b0bc0ee4b5d4d7c15.png)
formula | C_8H_20NO_6P Hill formula | C_8H_20NO_6P name | choline glycerophosphate IUPAC name | 2-[[(2S)-2, 3-dihydroxypropoxy]-oxido-phosphoryl]oxyethyl-trimethyl-azanium
Substance properties
molar mass | 257.22 g/mol phase | liquid (at STP) melting point | -98 °C boiling point | 64 °C density | 0.791 g/cm^3
Units
