Input interpretation
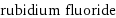
rubidium fluoride
Chemical names and formulas

formula | RbF Hill formula | FRb name | rubidium fluoride IUPAC name | rubidium(+1) cation fluoride alternate names | rubidium(+1) cation fluoride | rubidium monofluoride mass fractions | F (fluorine) 18.2% | Rb (rubidium) 81.8%
Structure diagram

Structure diagram
Basic properties
molar mass | 104.466 g/mol phase | solid (at STP) melting point | 753 °C boiling point | 1408 °C density | 3.557 g/cm^3
Units

Solid properties (at STP)

density | 3.557 g/cm^3 vapor pressure | 98.9 mmHg (at 1166 °C)
Units

Thermodynamic properties
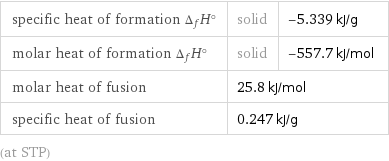
specific heat of formation Δ_fH° | solid | -5.339 kJ/g molar heat of formation Δ_fH° | solid | -557.7 kJ/mol molar heat of fusion | 25.8 kJ/mol | specific heat of fusion | 0.247 kJ/g | (at STP)
Chemical identifiers
![CAS number | 13446-74-7 PubChem CID number | 83473 PubChem SID number | 24855132 SMILES identifier | [F-].[Rb+] InChI identifier | InChI=1/FH.Rb/h1H;/q;+1/p-1/fF.Rb/h1h;/q-1;m RTECS number | VL8740000 MDL number | MFCD00011191](../image_source/453d153d5562fe04babf48975a7f7efd.png)
CAS number | 13446-74-7 PubChem CID number | 83473 PubChem SID number | 24855132 SMILES identifier | [F-].[Rb+] InChI identifier | InChI=1/FH.Rb/h1H;/q;+1/p-1/fF.Rb/h1h;/q-1;m RTECS number | VL8740000 MDL number | MFCD00011191
Toxicity properties

RTECS classes | other