Input interpretation
sodium metaborate
Chemical names and formulas

formula | BNaO_2 name | sodium metaborate IUPAC name | sodium oxido-oxo-borane alternate names | boric acid, monosodium salt | borosoap | kodalk | sodium borate mass fractions | B (boron) 16.4% | Na (sodium) 34.9% | O (oxygen) 48.6%
Structure diagram
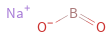
Structure diagram
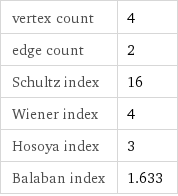
vertex count | 4 edge count | 2 Schultz index | 16 Wiener index | 4 Hosoya index | 3 Balaban index | 1.633
Basic properties
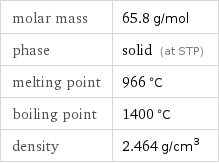
molar mass | 65.8 g/mol phase | solid (at STP) melting point | 966 °C boiling point | 1400 °C density | 2.464 g/cm^3
Units

Solid properties (at STP)

density | 2.464 g/cm^3 vapor pressure | 4×10^-8 mmHg (at 25 °C)
Units

Thermodynamic properties
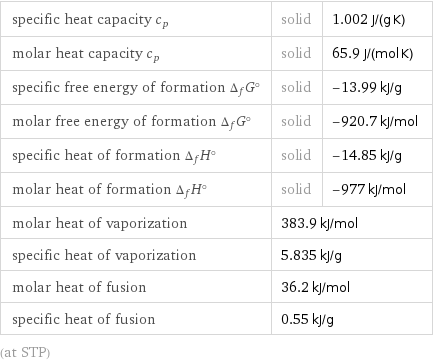
specific heat capacity c_p | solid | 1.002 J/(g K) molar heat capacity c_p | solid | 65.9 J/(mol K) specific free energy of formation Δ_fG° | solid | -13.99 kJ/g molar free energy of formation Δ_fG° | solid | -920.7 kJ/mol specific heat of formation Δ_fH° | solid | -14.85 kJ/g molar heat of formation Δ_fH° | solid | -977 kJ/mol molar heat of vaporization | 383.9 kJ/mol | specific heat of vaporization | 5.835 kJ/g | molar heat of fusion | 36.2 kJ/mol | specific heat of fusion | 0.55 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7775-19-1 PubChem CID number | 24491 SMILES identifier | B(=O)[O-].[Na+] InChI identifier | InChI=1/BO2.Na/c2-1-3;/q-1;+1 EU number | 231-891-6 Gmelin number | 38326 RTECS number | ED4640000](../image_source/d91d86d9835d64056231ec102aa12758.png)
CAS number | 7775-19-1 PubChem CID number | 24491 SMILES identifier | B(=O)[O-].[Na+] InChI identifier | InChI=1/BO2.Na/c2-1-3;/q-1;+1 EU number | 231-891-6 Gmelin number | 38326 RTECS number | ED4640000
Toxicity properties

RTECS classes | agricultural chemical and pesticide