Input interpretation
carbon (chemical element) | iodine (chemical element) | cadmium (chemical element)
Periodic table location
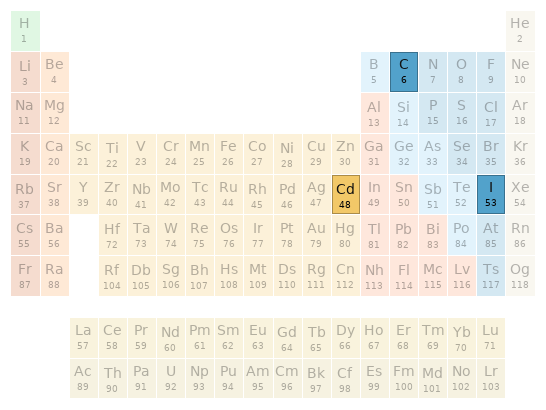
Periodic table location
Images

Images
Basic elemental properties
![| carbon | iodine | cadmium atomic symbol | C | I | Cd atomic number | 6 | 53 | 48 short electronic configuration | [He]2s^22p^2 | [Kr]5s^24d^105p^5 | [Kr]5s^24d^10 Aufbau diagram | 2p 2s | 5p 4d 5s | 4d 5s block | p | p | d group | 14 | 17 | 12 period | 2 | 5 | 5 atomic mass | 12.011 u | 126.90447 u | 112.414 u half-life | (stable) | (stable) | (stable)](../image_source/15c8b1a5a7edbe6f855b3e21fc4882e7.png)
| carbon | iodine | cadmium atomic symbol | C | I | Cd atomic number | 6 | 53 | 48 short electronic configuration | [He]2s^22p^2 | [Kr]5s^24d^105p^5 | [Kr]5s^24d^10 Aufbau diagram | 2p 2s | 5p 4d 5s | 4d 5s block | p | p | d group | 14 | 17 | 12 period | 2 | 5 | 5 atomic mass | 12.011 u | 126.90447 u | 112.414 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
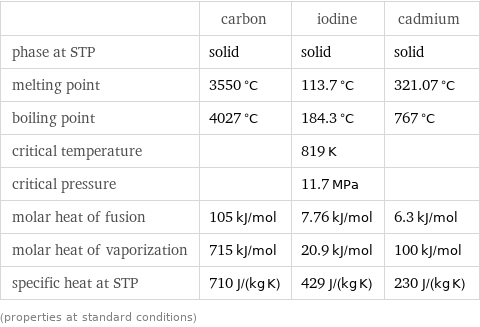
| carbon | iodine | cadmium phase at STP | solid | solid | solid melting point | 3550 °C | 113.7 °C | 321.07 °C boiling point | 4027 °C | 184.3 °C | 767 °C critical temperature | | 819 K | critical pressure | | 11.7 MPa | molar heat of fusion | 105 kJ/mol | 7.76 kJ/mol | 6.3 kJ/mol molar heat of vaporization | 715 kJ/mol | 20.9 kJ/mol | 100 kJ/mol specific heat at STP | 710 J/(kg K) | 429 J/(kg K) | 230 J/(kg K) (properties at standard conditions)
Material properties
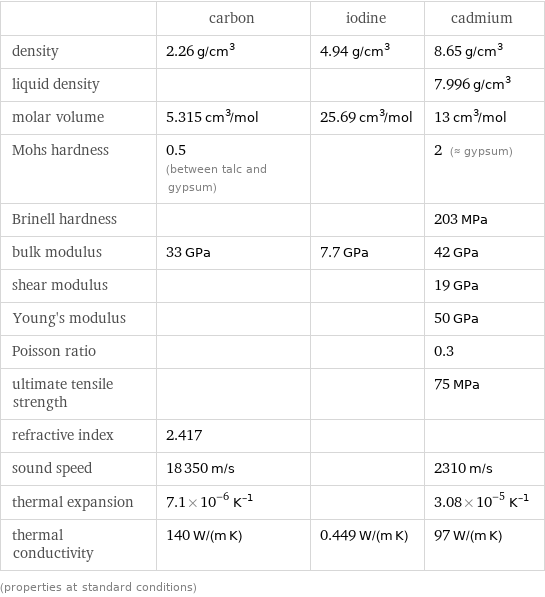
| carbon | iodine | cadmium density | 2.26 g/cm^3 | 4.94 g/cm^3 | 8.65 g/cm^3 liquid density | | | 7.996 g/cm^3 molar volume | 5.315 cm^3/mol | 25.69 cm^3/mol | 13 cm^3/mol Mohs hardness | 0.5 (between talc and gypsum) | | 2 (≈ gypsum) Brinell hardness | | | 203 MPa bulk modulus | 33 GPa | 7.7 GPa | 42 GPa shear modulus | | | 19 GPa Young's modulus | | | 50 GPa Poisson ratio | | | 0.3 ultimate tensile strength | | | 75 MPa refractive index | 2.417 | | sound speed | 18350 m/s | | 2310 m/s thermal expansion | 7.1×10^-6 K^(-1) | | 3.08×10^-5 K^(-1) thermal conductivity | 140 W/(m K) | 0.449 W/(m K) | 97 W/(m K) (properties at standard conditions)
Electromagnetic properties
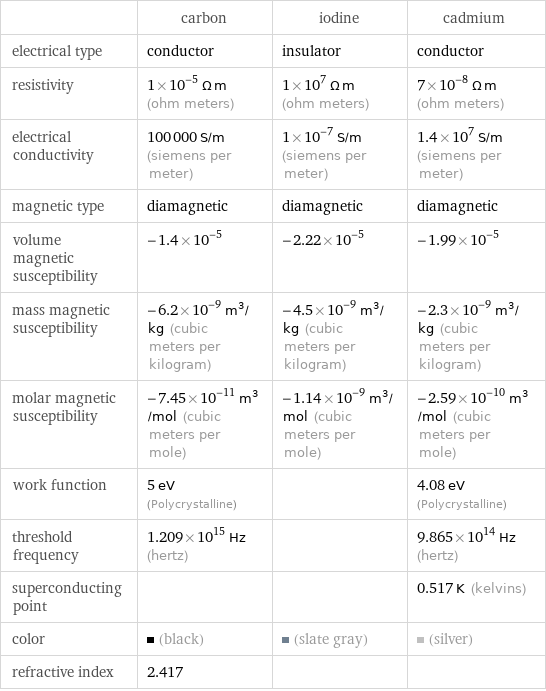
| carbon | iodine | cadmium electrical type | conductor | insulator | conductor resistivity | 1×10^-5 Ω m (ohm meters) | 1×10^7 Ω m (ohm meters) | 7×10^-8 Ω m (ohm meters) electrical conductivity | 100000 S/m (siemens per meter) | 1×10^-7 S/m (siemens per meter) | 1.4×10^7 S/m (siemens per meter) magnetic type | diamagnetic | diamagnetic | diamagnetic volume magnetic susceptibility | -1.4×10^-5 | -2.22×10^-5 | -1.99×10^-5 mass magnetic susceptibility | -6.2×10^-9 m^3/kg (cubic meters per kilogram) | -4.5×10^-9 m^3/kg (cubic meters per kilogram) | -2.3×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -7.45×10^-11 m^3/mol (cubic meters per mole) | -1.14×10^-9 m^3/mol (cubic meters per mole) | -2.59×10^-10 m^3/mol (cubic meters per mole) work function | 5 eV (Polycrystalline) | | 4.08 eV (Polycrystalline) threshold frequency | 1.209×10^15 Hz (hertz) | | 9.865×10^14 Hz (hertz) superconducting point | | | 0.517 K (kelvins) color | (black) | (slate gray) | (silver) refractive index | 2.417 | |
Reactivity
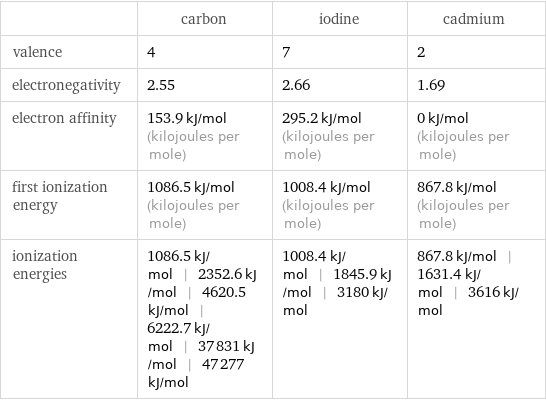
| carbon | iodine | cadmium valence | 4 | 7 | 2 electronegativity | 2.55 | 2.66 | 1.69 electron affinity | 153.9 kJ/mol (kilojoules per mole) | 295.2 kJ/mol (kilojoules per mole) | 0 kJ/mol (kilojoules per mole) first ionization energy | 1086.5 kJ/mol (kilojoules per mole) | 1008.4 kJ/mol (kilojoules per mole) | 867.8 kJ/mol (kilojoules per mole) ionization energies | 1086.5 kJ/mol | 2352.6 kJ/mol | 4620.5 kJ/mol | 6222.7 kJ/mol | 37831 kJ/mol | 47277 kJ/mol | 1008.4 kJ/mol | 1845.9 kJ/mol | 3180 kJ/mol | 867.8 kJ/mol | 1631.4 kJ/mol | 3616 kJ/mol
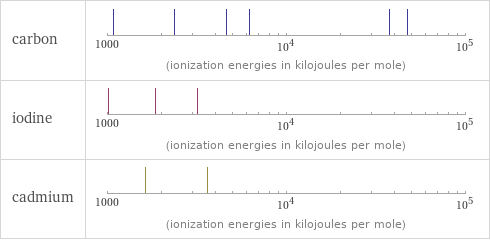
Reactivity
Atomic properties

| carbon | iodine | cadmium term symbol | ^3P_0 | ^2P_(3/2) | ^1S_0 atomic radius | 70 pm | 140 pm | 155 pm covalent radius | 76 pm | 139 pm | 144 pm van der Waals radius | 170 pm | 198 pm | 158 pm (electronic ground state properties)
Abundances
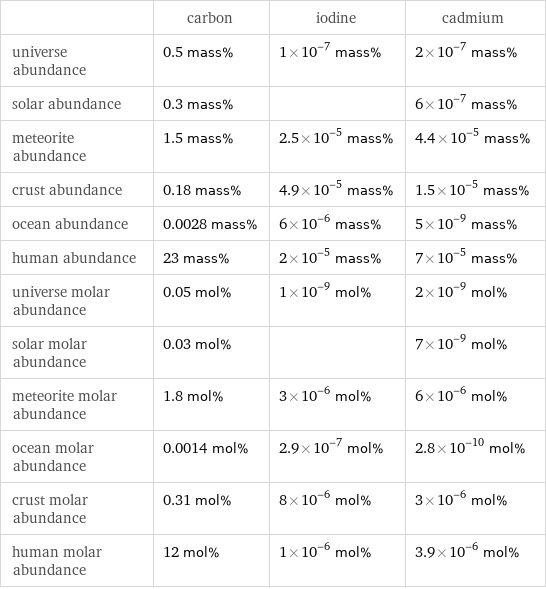
| carbon | iodine | cadmium universe abundance | 0.5 mass% | 1×10^-7 mass% | 2×10^-7 mass% solar abundance | 0.3 mass% | | 6×10^-7 mass% meteorite abundance | 1.5 mass% | 2.5×10^-5 mass% | 4.4×10^-5 mass% crust abundance | 0.18 mass% | 4.9×10^-5 mass% | 1.5×10^-5 mass% ocean abundance | 0.0028 mass% | 6×10^-6 mass% | 5×10^-9 mass% human abundance | 23 mass% | 2×10^-5 mass% | 7×10^-5 mass% universe molar abundance | 0.05 mol% | 1×10^-9 mol% | 2×10^-9 mol% solar molar abundance | 0.03 mol% | | 7×10^-9 mol% meteorite molar abundance | 1.8 mol% | 3×10^-6 mol% | 6×10^-6 mol% ocean molar abundance | 0.0014 mol% | 2.9×10^-7 mol% | 2.8×10^-10 mol% crust molar abundance | 0.31 mol% | 8×10^-6 mol% | 3×10^-6 mol% human molar abundance | 12 mol% | 1×10^-6 mol% | 3.9×10^-6 mol%