Input interpretation
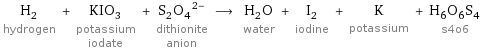
H_2 hydrogen + KIO_3 potassium iodate + (S_2O_4)^(2-) dithionite anion ⟶ H_2O water + I_2 iodine + K potassium + H_6O_6S_4 s4o6
Chemical names and formulas
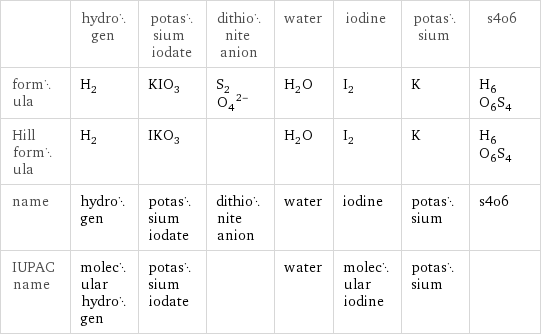
| hydrogen | potassium iodate | dithionite anion | water | iodine | potassium | s4o6 formula | H_2 | KIO_3 | (S_2O_4)^(2-) | H_2O | I_2 | K | H_6O_6S_4 Hill formula | H_2 | IKO_3 | | H_2O | I_2 | K | H_6O_6S_4 name | hydrogen | potassium iodate | dithionite anion | water | iodine | potassium | s4o6 IUPAC name | molecular hydrogen | potassium iodate | | water | molecular iodine | potassium |