Input interpretation
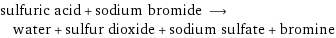
sulfuric acid + sodium bromide ⟶ water + sulfur dioxide + sodium sulfate + bromine
Balanced equation

Balance the chemical equation algebraically: + ⟶ + + + Add stoichiometric coefficients, c_i, to the reactants and products: c_1 + c_2 ⟶ c_3 + c_4 + c_5 + c_6 Set the number of atoms in the reactants equal to the number of atoms in the products for H, O, S, Br and Na: H: | 2 c_1 = 2 c_3 O: | 4 c_1 = c_3 + 2 c_4 + 4 c_5 S: | c_1 = c_4 + c_5 Br: | c_2 = 2 c_6 Na: | c_2 = 2 c_5 Since the coefficients are relative quantities and underdetermined, choose a coefficient to set arbitrarily. To keep the coefficients small, the arbitrary value is ordinarily one. For instance, set c_4 = 1 and solve the system of equations for the remaining coefficients: c_1 = 2 c_2 = 2 c_3 = 2 c_4 = 1 c_5 = 1 c_6 = 1 Substitute the coefficients into the chemical reaction to obtain the balanced equation: Answer: | | 2 + 2 ⟶ 2 + + +
Structures
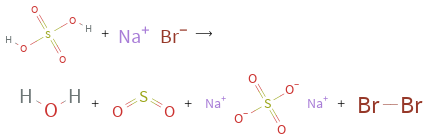
+ ⟶ + + +
Names
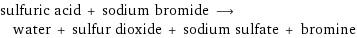
sulfuric acid + sodium bromide ⟶ water + sulfur dioxide + sodium sulfate + bromine
Reaction thermodynamics
Enthalpy
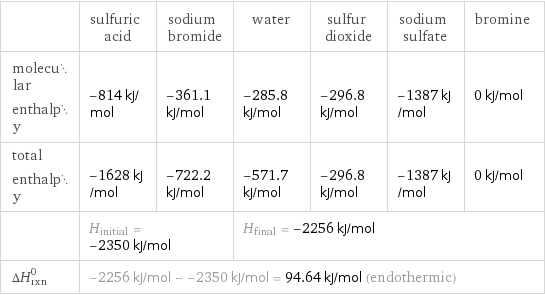
| sulfuric acid | sodium bromide | water | sulfur dioxide | sodium sulfate | bromine molecular enthalpy | -814 kJ/mol | -361.1 kJ/mol | -285.8 kJ/mol | -296.8 kJ/mol | -1387 kJ/mol | 0 kJ/mol total enthalpy | -1628 kJ/mol | -722.2 kJ/mol | -571.7 kJ/mol | -296.8 kJ/mol | -1387 kJ/mol | 0 kJ/mol | H_initial = -2350 kJ/mol | | H_final = -2256 kJ/mol | | | ΔH_rxn^0 | -2256 kJ/mol - -2350 kJ/mol = 94.64 kJ/mol (endothermic) | | | | |
Gibbs free energy
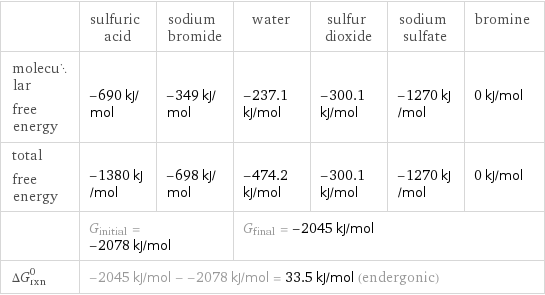
| sulfuric acid | sodium bromide | water | sulfur dioxide | sodium sulfate | bromine molecular free energy | -690 kJ/mol | -349 kJ/mol | -237.1 kJ/mol | -300.1 kJ/mol | -1270 kJ/mol | 0 kJ/mol total free energy | -1380 kJ/mol | -698 kJ/mol | -474.2 kJ/mol | -300.1 kJ/mol | -1270 kJ/mol | 0 kJ/mol | G_initial = -2078 kJ/mol | | G_final = -2045 kJ/mol | | | ΔG_rxn^0 | -2045 kJ/mol - -2078 kJ/mol = 33.5 kJ/mol (endergonic) | | | | |
Chemical names and formulas
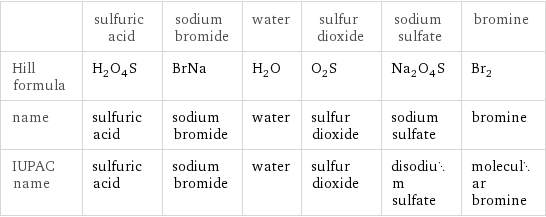
| sulfuric acid | sodium bromide | water | sulfur dioxide | sodium sulfate | bromine Hill formula | H_2O_4S | BrNa | H_2O | O_2S | Na_2O_4S | Br_2 name | sulfuric acid | sodium bromide | water | sulfur dioxide | sodium sulfate | bromine IUPAC name | sulfuric acid | sodium bromide | water | sulfur dioxide | disodium sulfate | molecular bromine