Input interpretation
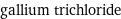
gallium trichloride
Chemical names and formulas
formula | GaCl_3 Hill formula | Cl_3Ga name | gallium trichloride IUPAC name | trichlorogallane alternate names | gallium(3+) chloride | gallium chloride | gallium(III) chloride | trichlorogallane mass fractions | Cl (chlorine) 60.4% | Ga (gallium) 39.6%
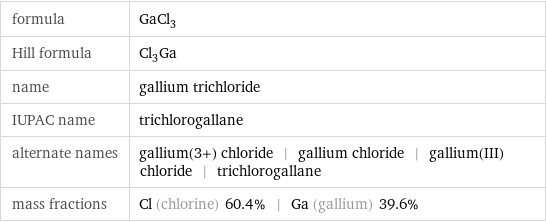
Structure diagram
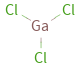
Structure diagram

| bond counts | bond lengths | 3 bonds | 2.1 Å
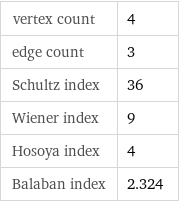
vertex count | 4 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
3D structure

3D structure
Basic properties
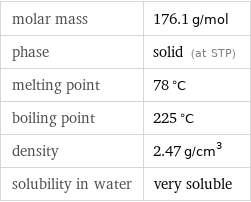
molar mass | 176.1 g/mol phase | solid (at STP) melting point | 78 °C boiling point | 225 °C density | 2.47 g/cm^3 solubility in water | very soluble
Units

Solid properties (at STP)
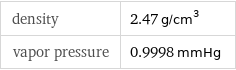
density | 2.47 g/cm^3 vapor pressure | 0.9998 mmHg
Units

Thermodynamic properties
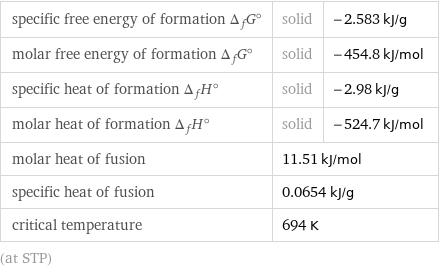
specific free energy of formation Δ_fG° | solid | -2.583 kJ/g molar free energy of formation Δ_fG° | solid | -454.8 kJ/mol specific heat of formation Δ_fH° | solid | -2.98 kJ/g molar heat of formation Δ_fH° | solid | -524.7 kJ/mol molar heat of fusion | 11.51 kJ/mol | specific heat of fusion | 0.0654 kJ/g | critical temperature | 694 K | (at STP)
Chemical identifiers
Cl InChI identifier | InChI=1/3ClH.Ga/h3*1H;/q;;;+3/p-3/f3Cl.Ga/h3*1h;/q3*-1;m RTECS number | LW9100000 MDL number | MFCD00011018](../image_source/13ed60158f7673cc9d2647d8ed9f348a.png)
CAS number | 13450-90-3 PubChem CID number | 26010 SMILES identifier | Cl[Ga](Cl)Cl InChI identifier | InChI=1/3ClH.Ga/h3*1H;/q;;;+3/p-3/f3Cl.Ga/h3*1h;/q3*-1;m RTECS number | LW9100000 MDL number | MFCD00011018
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 1
Toxicity properties

RTECS classes | mutagen