Input interpretation

insulators | molar mass
Summary
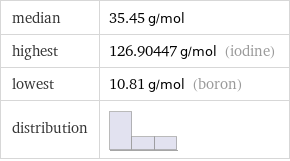
median | 35.45 g/mol highest | 126.90447 g/mol (iodine) lowest | 10.81 g/mol (boron) distribution |
Units

Ranked values
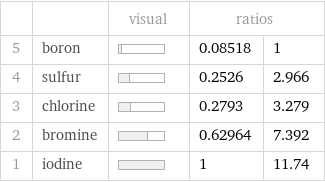
| | visual | ratios | 5 | boron | | 0.08518 | 1 4 | sulfur | | 0.2526 | 2.966 3 | chlorine | | 0.2793 | 3.279 2 | bromine | | 0.62964 | 7.392 1 | iodine | | 1 | 11.74
Molar mass rankings
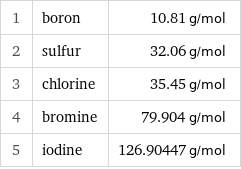
1 | boron | 10.81 g/mol 2 | sulfur | 32.06 g/mol 3 | chlorine | 35.45 g/mol 4 | bromine | 79.904 g/mol 5 | iodine | 126.90447 g/mol
Unit conversion for median molar mass 35.45 g/mol
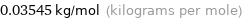
0.03545 kg/mol (kilograms per mole)
Comparisons for median molar mass 35.45 g/mol
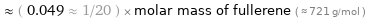
≈ ( 0.049 ≈ 1/20 ) × molar mass of fullerene ( ≈ 721 g/mol )
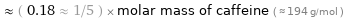
≈ ( 0.18 ≈ 1/5 ) × molar mass of caffeine ( ≈ 194 g/mol )
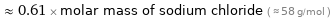
≈ 0.61 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
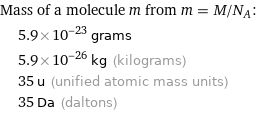
Mass of a molecule m from m = M/N_A: | 5.9×10^-23 grams | 5.9×10^-26 kg (kilograms) | 35 u (unified atomic mass units) | 35 Da (daltons)
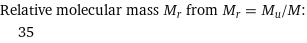
Relative molecular mass M_r from M_r = M_u/M: | 35