Input interpretation

period 5 elements | atomic mass
Summary

median | 104.66 u highest | 131.293 u (xenon) lowest | 85.4678 u (rubidium) distribution |
Units

Distribution plots
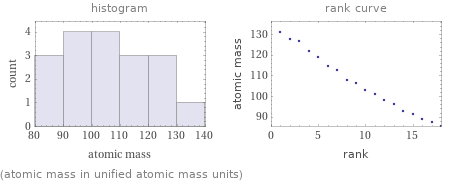
(atomic mass in unified atomic mass units)
Atomic mass rankings
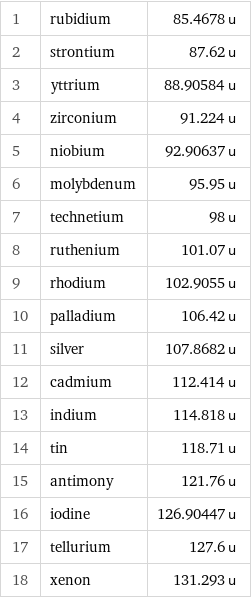
1 | rubidium | 85.4678 u 2 | strontium | 87.62 u 3 | yttrium | 88.90584 u 4 | zirconium | 91.224 u 5 | niobium | 92.90637 u 6 | molybdenum | 95.95 u 7 | technetium | 98 u 8 | ruthenium | 101.07 u 9 | rhodium | 102.9055 u 10 | palladium | 106.42 u 11 | silver | 107.8682 u 12 | cadmium | 112.414 u 13 | indium | 114.818 u 14 | tin | 118.71 u 15 | antimony | 121.76 u 16 | iodine | 126.90447 u 17 | tellurium | 127.6 u 18 | xenon | 131.293 u
Unit conversions for median atomic mass 104.66 u
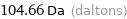
104.66 Da (daltons)

0.10466 kDa (kilodaltons)
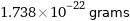
1.738×10^-22 grams

1.738×10^-25 kg (kilograms)

104.67 chemical atomic mass units (unit officially deprecated)
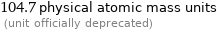
104.7 physical atomic mass units (unit officially deprecated)
Corresponding quantities
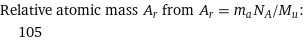
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 105
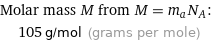
Molar mass M from M = m_aN_A: | 105 g/mol (grams per mole)