Input interpretation

homonuclear diatomic molecules | molar heat of fusion
Summary
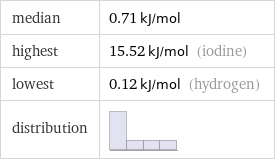
median | 0.71 kJ/mol highest | 15.52 kJ/mol (iodine) lowest | 0.12 kJ/mol (hydrogen) distribution |
Units

Ranked values
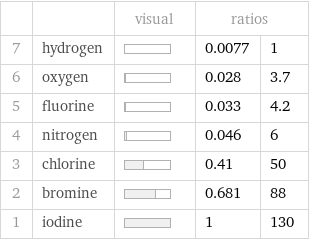
| | visual | ratios | 7 | hydrogen | | 0.0077 | 1 6 | oxygen | | 0.028 | 3.7 5 | fluorine | | 0.033 | 4.2 4 | nitrogen | | 0.046 | 6 3 | chlorine | | 0.41 | 50 2 | bromine | | 0.681 | 88 1 | iodine | | 1 | 130
Molar heat of fusion rankings
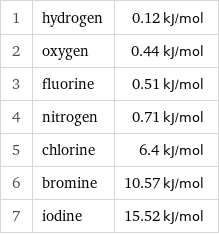
1 | hydrogen | 0.12 kJ/mol 2 | oxygen | 0.44 kJ/mol 3 | fluorine | 0.51 kJ/mol 4 | nitrogen | 0.71 kJ/mol 5 | chlorine | 6.4 kJ/mol 6 | bromine | 10.57 kJ/mol 7 | iodine | 15.52 kJ/mol
Unit conversions for median molar heat of fusion 0.71 kJ/mol
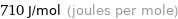
710 J/mol (joules per mole)
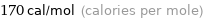
170 cal/mol (calories per mole)

0.17 kcal/mol (thermochemical kilocalories per mole)