Input interpretation
iodine (chemical element) | gold (chemical element) | indium (chemical element)
Periodic table location
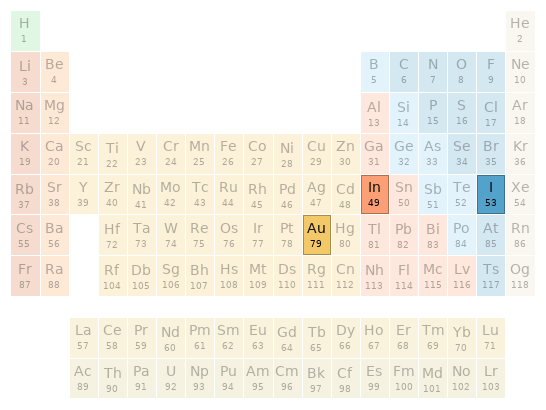
Periodic table location
Images
Images
Basic elemental properties
![| iodine | gold | indium atomic symbol | I | Au | In atomic number | 53 | 79 | 49 short electronic configuration | [Kr]5s^24d^105p^5 | [Xe]6s^14f^145d^10 | [Kr]5s^24d^105p^1 Aufbau diagram | 5p 4d 5s | 5d 4f 6s | 5p 4d 5s block | p | d | p group | 17 | 11 | 13 period | 5 | 6 | 5 atomic mass | 126.90447 u | 196.966569 u | 114.818 u half-life | (stable) | (stable) | (stable)](../image_source/2412e22b293700bb5ef042da2592142c.png)
| iodine | gold | indium atomic symbol | I | Au | In atomic number | 53 | 79 | 49 short electronic configuration | [Kr]5s^24d^105p^5 | [Xe]6s^14f^145d^10 | [Kr]5s^24d^105p^1 Aufbau diagram | 5p 4d 5s | 5d 4f 6s | 5p 4d 5s block | p | d | p group | 17 | 11 | 13 period | 5 | 6 | 5 atomic mass | 126.90447 u | 196.966569 u | 114.818 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
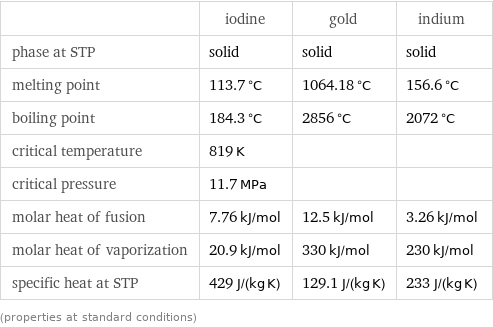
| iodine | gold | indium phase at STP | solid | solid | solid melting point | 113.7 °C | 1064.18 °C | 156.6 °C boiling point | 184.3 °C | 2856 °C | 2072 °C critical temperature | 819 K | | critical pressure | 11.7 MPa | | molar heat of fusion | 7.76 kJ/mol | 12.5 kJ/mol | 3.26 kJ/mol molar heat of vaporization | 20.9 kJ/mol | 330 kJ/mol | 230 kJ/mol specific heat at STP | 429 J/(kg K) | 129.1 J/(kg K) | 233 J/(kg K) (properties at standard conditions)
Material properties
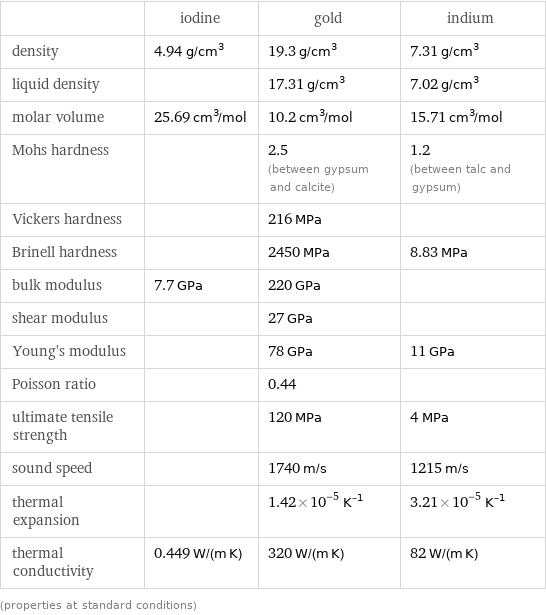
| iodine | gold | indium density | 4.94 g/cm^3 | 19.3 g/cm^3 | 7.31 g/cm^3 liquid density | | 17.31 g/cm^3 | 7.02 g/cm^3 molar volume | 25.69 cm^3/mol | 10.2 cm^3/mol | 15.71 cm^3/mol Mohs hardness | | 2.5 (between gypsum and calcite) | 1.2 (between talc and gypsum) Vickers hardness | | 216 MPa | Brinell hardness | | 2450 MPa | 8.83 MPa bulk modulus | 7.7 GPa | 220 GPa | shear modulus | | 27 GPa | Young's modulus | | 78 GPa | 11 GPa Poisson ratio | | 0.44 | ultimate tensile strength | | 120 MPa | 4 MPa sound speed | | 1740 m/s | 1215 m/s thermal expansion | | 1.42×10^-5 K^(-1) | 3.21×10^-5 K^(-1) thermal conductivity | 0.449 W/(m K) | 320 W/(m K) | 82 W/(m K) (properties at standard conditions)
Electromagnetic properties
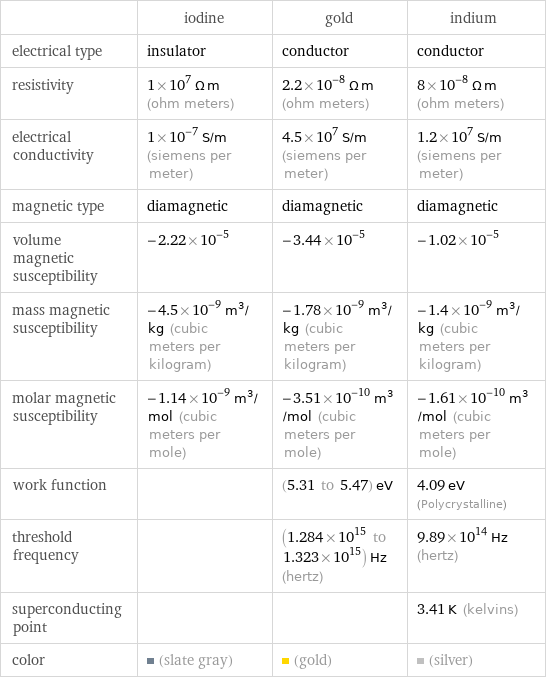
| iodine | gold | indium electrical type | insulator | conductor | conductor resistivity | 1×10^7 Ω m (ohm meters) | 2.2×10^-8 Ω m (ohm meters) | 8×10^-8 Ω m (ohm meters) electrical conductivity | 1×10^-7 S/m (siemens per meter) | 4.5×10^7 S/m (siemens per meter) | 1.2×10^7 S/m (siemens per meter) magnetic type | diamagnetic | diamagnetic | diamagnetic volume magnetic susceptibility | -2.22×10^-5 | -3.44×10^-5 | -1.02×10^-5 mass magnetic susceptibility | -4.5×10^-9 m^3/kg (cubic meters per kilogram) | -1.78×10^-9 m^3/kg (cubic meters per kilogram) | -1.4×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -1.14×10^-9 m^3/mol (cubic meters per mole) | -3.51×10^-10 m^3/mol (cubic meters per mole) | -1.61×10^-10 m^3/mol (cubic meters per mole) work function | | (5.31 to 5.47) eV | 4.09 eV (Polycrystalline) threshold frequency | | (1.284×10^15 to 1.323×10^15) Hz (hertz) | 9.89×10^14 Hz (hertz) superconducting point | | | 3.41 K (kelvins) color | (slate gray) | (gold) | (silver)
Reactivity
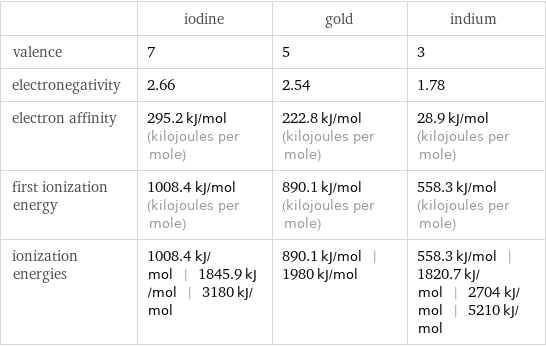
| iodine | gold | indium valence | 7 | 5 | 3 electronegativity | 2.66 | 2.54 | 1.78 electron affinity | 295.2 kJ/mol (kilojoules per mole) | 222.8 kJ/mol (kilojoules per mole) | 28.9 kJ/mol (kilojoules per mole) first ionization energy | 1008.4 kJ/mol (kilojoules per mole) | 890.1 kJ/mol (kilojoules per mole) | 558.3 kJ/mol (kilojoules per mole) ionization energies | 1008.4 kJ/mol | 1845.9 kJ/mol | 3180 kJ/mol | 890.1 kJ/mol | 1980 kJ/mol | 558.3 kJ/mol | 1820.7 kJ/mol | 2704 kJ/mol | 5210 kJ/mol
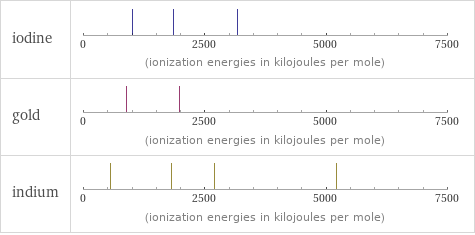
Reactivity
Atomic properties
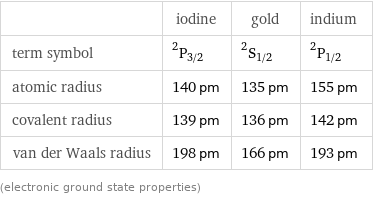
| iodine | gold | indium term symbol | ^2P_(3/2) | ^2S_(1/2) | ^2P_(1/2) atomic radius | 140 pm | 135 pm | 155 pm covalent radius | 139 pm | 136 pm | 142 pm van der Waals radius | 198 pm | 166 pm | 193 pm (electronic ground state properties)
Abundances
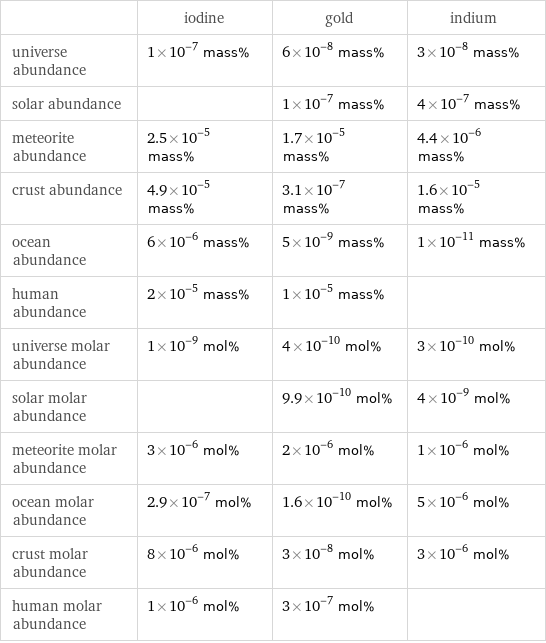
| iodine | gold | indium universe abundance | 1×10^-7 mass% | 6×10^-8 mass% | 3×10^-8 mass% solar abundance | | 1×10^-7 mass% | 4×10^-7 mass% meteorite abundance | 2.5×10^-5 mass% | 1.7×10^-5 mass% | 4.4×10^-6 mass% crust abundance | 4.9×10^-5 mass% | 3.1×10^-7 mass% | 1.6×10^-5 mass% ocean abundance | 6×10^-6 mass% | 5×10^-9 mass% | 1×10^-11 mass% human abundance | 2×10^-5 mass% | 1×10^-5 mass% | universe molar abundance | 1×10^-9 mol% | 4×10^-10 mol% | 3×10^-10 mol% solar molar abundance | | 9.9×10^-10 mol% | 4×10^-9 mol% meteorite molar abundance | 3×10^-6 mol% | 2×10^-6 mol% | 1×10^-6 mol% ocean molar abundance | 2.9×10^-7 mol% | 1.6×10^-10 mol% | 5×10^-6 mol% crust molar abundance | 8×10^-6 mol% | 3×10^-8 mol% | 3×10^-6 mol% human molar abundance | 1×10^-6 mol% | 3×10^-7 mol% |