Input interpretation
anions
Members

hydride anion | hydroxide anion | oxide anion | cyanide anion | fluoride anion | chloride anion | bromide anion | iodide anion | acetate anion | borate anion | carbonate anion | hydrogen carbonate anion | nitrate anion | phosphate anion | sulfate anion | chlorite anion | chlorate anion | chromate anion | dichromate anion | manganate anion (total: 20)
Ionic radii
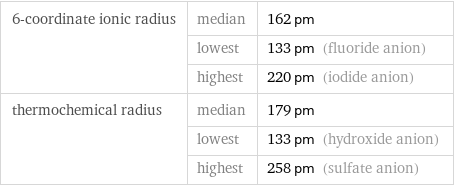
6-coordinate ionic radius | median | 162 pm | lowest | 133 pm (fluoride anion) | highest | 220 pm (iodide anion) thermochemical radius | median | 179 pm | lowest | 133 pm (hydroxide anion) | highest | 258 pm (sulfate anion)
Units
