Input interpretation
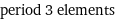
period 3 elements
Members
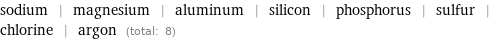
sodium | magnesium | aluminum | silicon | phosphorus | sulfur | chlorine | argon (total: 8)
Periodic table location
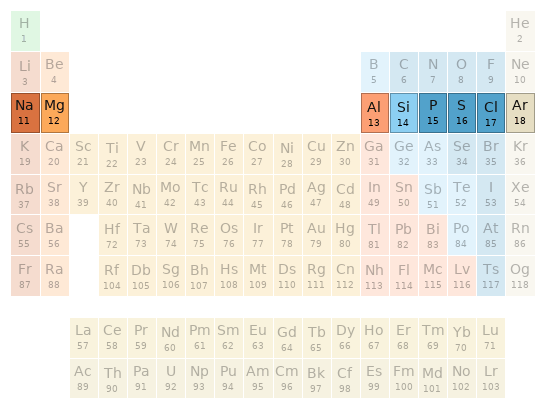
Periodic table location
Images
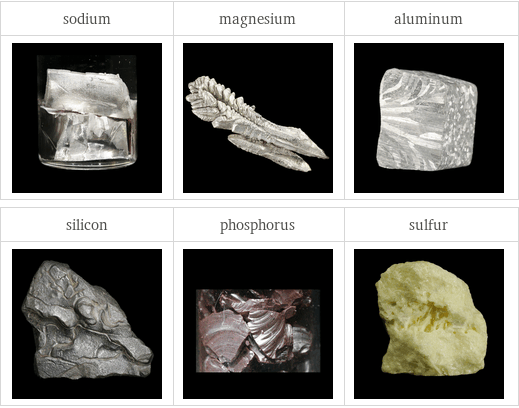
Images
Basic elemental properties
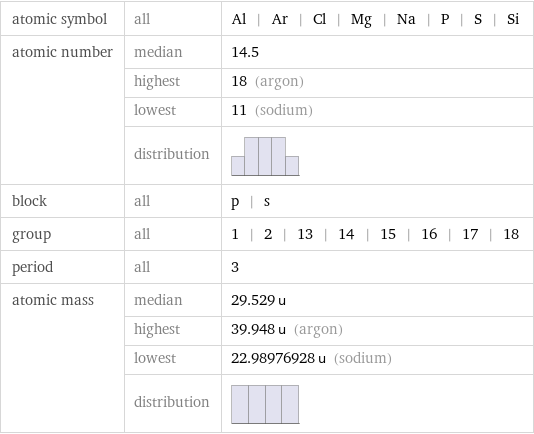
atomic symbol | all | Al | Ar | Cl | Mg | Na | P | S | Si atomic number | median | 14.5 | highest | 18 (argon) | lowest | 11 (sodium) | distribution | block | all | p | s group | all | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 period | all | 3 atomic mass | median | 29.529 u | highest | 39.948 u (argon) | lowest | 22.98976928 u (sodium) | distribution |
Thermodynamic properties
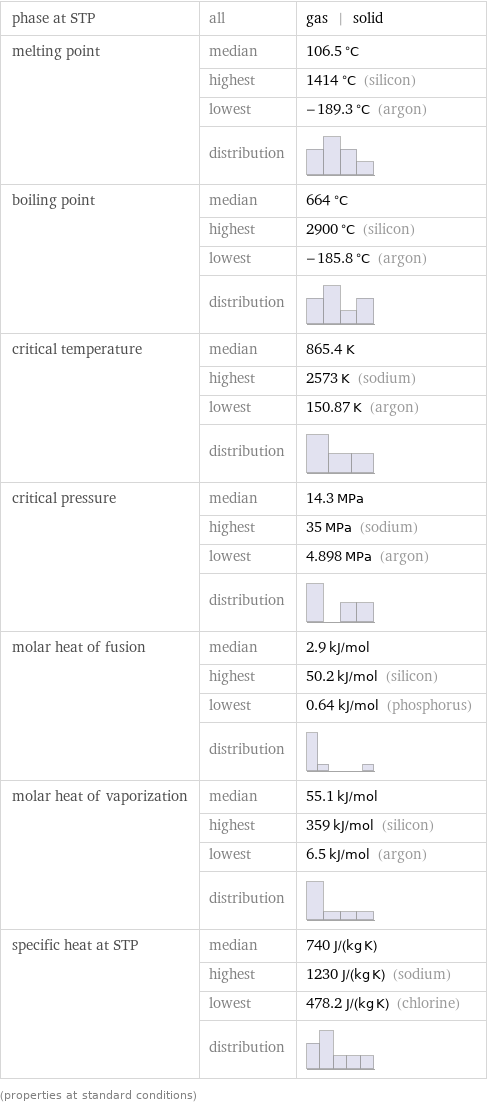
phase at STP | all | gas | solid melting point | median | 106.5 °C | highest | 1414 °C (silicon) | lowest | -189.3 °C (argon) | distribution | boiling point | median | 664 °C | highest | 2900 °C (silicon) | lowest | -185.8 °C (argon) | distribution | critical temperature | median | 865.4 K | highest | 2573 K (sodium) | lowest | 150.87 K (argon) | distribution | critical pressure | median | 14.3 MPa | highest | 35 MPa (sodium) | lowest | 4.898 MPa (argon) | distribution | molar heat of fusion | median | 2.9 kJ/mol | highest | 50.2 kJ/mol (silicon) | lowest | 0.64 kJ/mol (phosphorus) | distribution | molar heat of vaporization | median | 55.1 kJ/mol | highest | 359 kJ/mol (silicon) | lowest | 6.5 kJ/mol (argon) | distribution | specific heat at STP | median | 740 J/(kg K) | highest | 1230 J/(kg K) (sodium) | lowest | 478.2 J/(kg K) (chlorine) | distribution | (properties at standard conditions)
Material properties
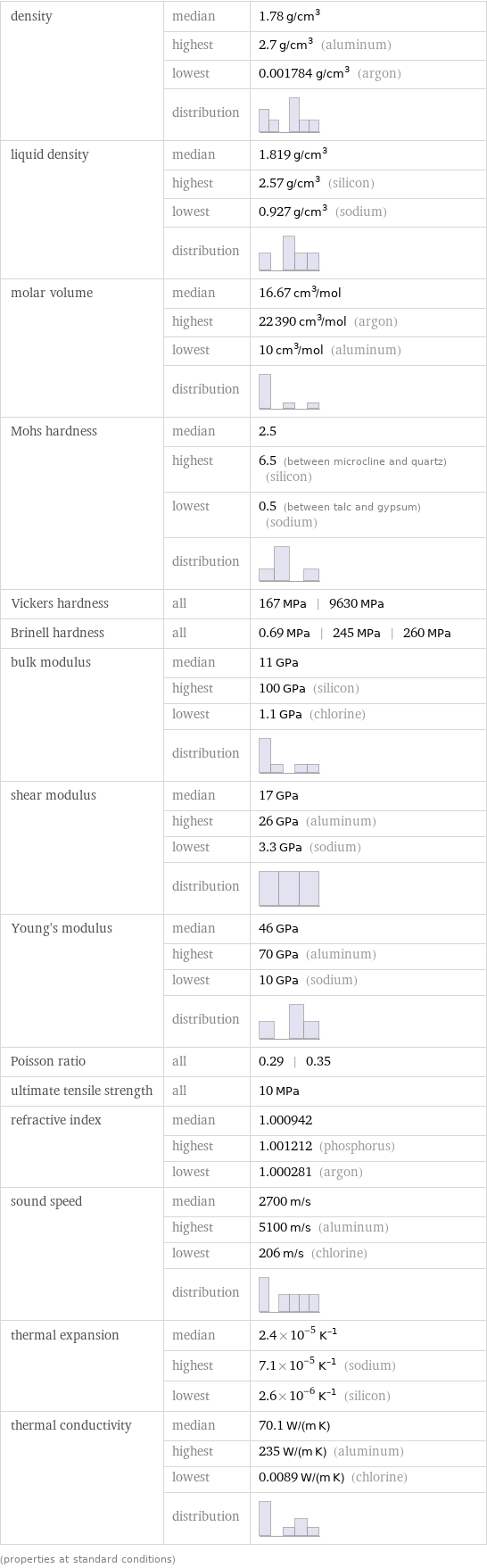
density | median | 1.78 g/cm^3 | highest | 2.7 g/cm^3 (aluminum) | lowest | 0.001784 g/cm^3 (argon) | distribution | liquid density | median | 1.819 g/cm^3 | highest | 2.57 g/cm^3 (silicon) | lowest | 0.927 g/cm^3 (sodium) | distribution | molar volume | median | 16.67 cm^3/mol | highest | 22390 cm^3/mol (argon) | lowest | 10 cm^3/mol (aluminum) | distribution | Mohs hardness | median | 2.5 | highest | 6.5 (between microcline and quartz) (silicon) | lowest | 0.5 (between talc and gypsum) (sodium) | distribution | Vickers hardness | all | 167 MPa | 9630 MPa Brinell hardness | all | 0.69 MPa | 245 MPa | 260 MPa bulk modulus | median | 11 GPa | highest | 100 GPa (silicon) | lowest | 1.1 GPa (chlorine) | distribution | shear modulus | median | 17 GPa | highest | 26 GPa (aluminum) | lowest | 3.3 GPa (sodium) | distribution | Young's modulus | median | 46 GPa | highest | 70 GPa (aluminum) | lowest | 10 GPa (sodium) | distribution | Poisson ratio | all | 0.29 | 0.35 ultimate tensile strength | all | 10 MPa refractive index | median | 1.000942 | highest | 1.001212 (phosphorus) | lowest | 1.000281 (argon) sound speed | median | 2700 m/s | highest | 5100 m/s (aluminum) | lowest | 206 m/s (chlorine) | distribution | thermal expansion | median | 2.4×10^-5 K^(-1) | highest | 7.1×10^-5 K^(-1) (sodium) | lowest | 2.6×10^-6 K^(-1) (silicon) thermal conductivity | median | 70.1 W/(m K) | highest | 235 W/(m K) (aluminum) | lowest | 0.0089 W/(m K) (chlorine) | distribution | (properties at standard conditions)
Electromagnetic properties
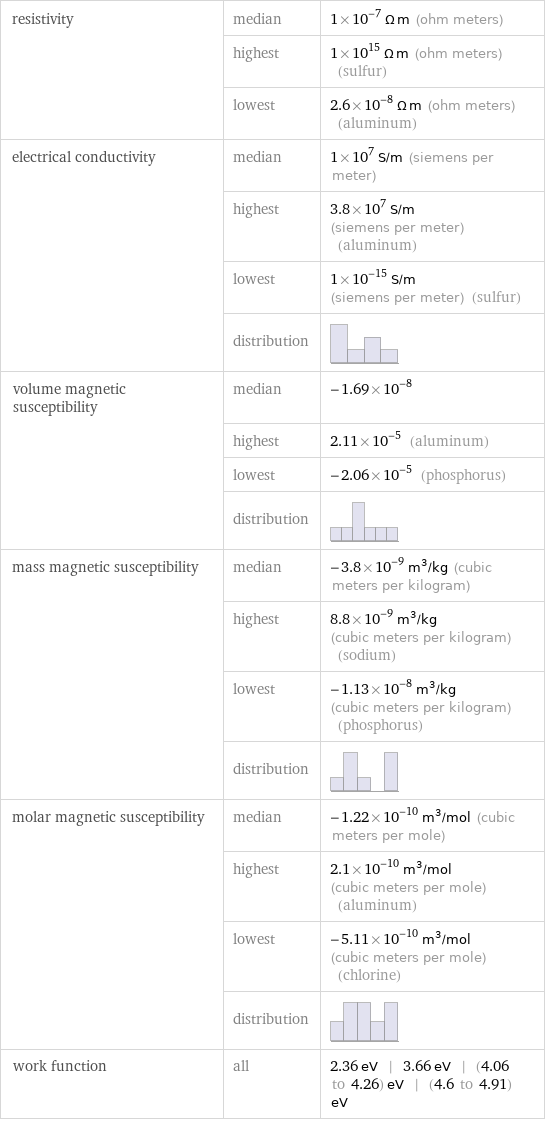
resistivity | median | 1×10^-7 Ω m (ohm meters) | highest | 1×10^15 Ω m (ohm meters) (sulfur) | lowest | 2.6×10^-8 Ω m (ohm meters) (aluminum) electrical conductivity | median | 1×10^7 S/m (siemens per meter) | highest | 3.8×10^7 S/m (siemens per meter) (aluminum) | lowest | 1×10^-15 S/m (siemens per meter) (sulfur) | distribution | volume magnetic susceptibility | median | -1.69×10^-8 | highest | 2.11×10^-5 (aluminum) | lowest | -2.06×10^-5 (phosphorus) | distribution | mass magnetic susceptibility | median | -3.8×10^-9 m^3/kg (cubic meters per kilogram) | highest | 8.8×10^-9 m^3/kg (cubic meters per kilogram) (sodium) | lowest | -1.13×10^-8 m^3/kg (cubic meters per kilogram) (phosphorus) | distribution | molar magnetic susceptibility | median | -1.22×10^-10 m^3/mol (cubic meters per mole) | highest | 2.1×10^-10 m^3/mol (cubic meters per mole) (aluminum) | lowest | -5.11×10^-10 m^3/mol (cubic meters per mole) (chlorine) | distribution | work function | all | 2.36 eV | 3.66 eV | (4.06 to 4.26) eV | (4.6 to 4.91) eV
Reactivity
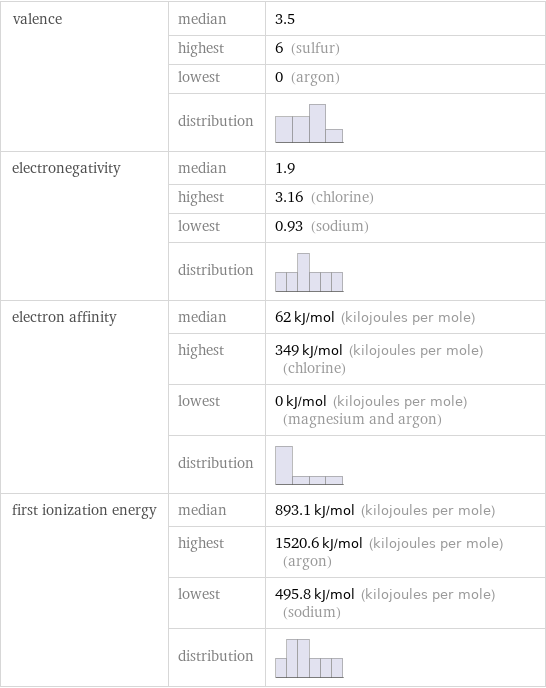
valence | median | 3.5 | highest | 6 (sulfur) | lowest | 0 (argon) | distribution | electronegativity | median | 1.9 | highest | 3.16 (chlorine) | lowest | 0.93 (sodium) | distribution | electron affinity | median | 62 kJ/mol (kilojoules per mole) | highest | 349 kJ/mol (kilojoules per mole) (chlorine) | lowest | 0 kJ/mol (kilojoules per mole) (magnesium and argon) | distribution | first ionization energy | median | 893.1 kJ/mol (kilojoules per mole) | highest | 1520.6 kJ/mol (kilojoules per mole) (argon) | lowest | 495.8 kJ/mol (kilojoules per mole) (sodium) | distribution |
Atomic properties
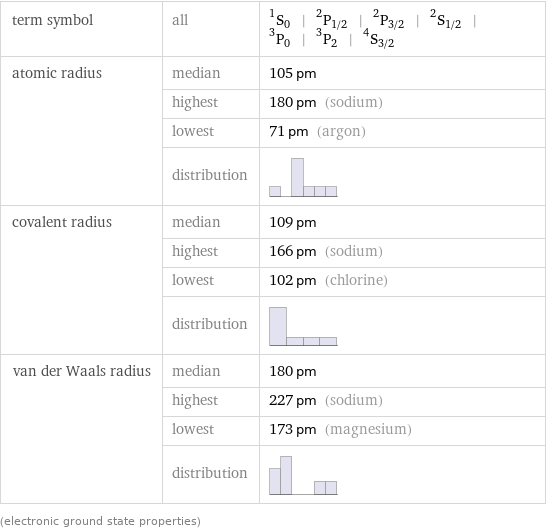
term symbol | all | ^1S_0 | ^2P_(1/2) | ^2P_(3/2) | ^2S_(1/2) | ^3P_0 | ^3P_2 | ^4S_(3/2) atomic radius | median | 105 pm | highest | 180 pm (sodium) | lowest | 71 pm (argon) | distribution | covalent radius | median | 109 pm | highest | 166 pm (sodium) | lowest | 102 pm (chlorine) | distribution | van der Waals radius | median | 180 pm | highest | 227 pm (sodium) | lowest | 173 pm (magnesium) | distribution | (electronic ground state properties)