Input interpretation
perchloric acid | lithium bromide
Chemical names and formulas
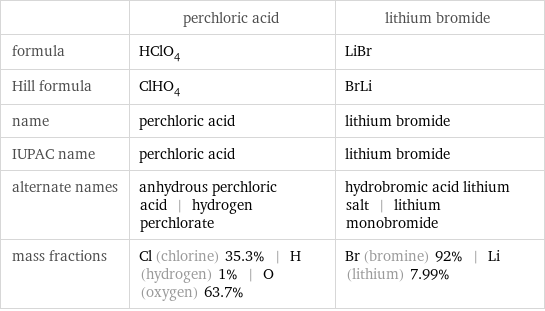
| perchloric acid | lithium bromide formula | HClO_4 | LiBr Hill formula | ClHO_4 | BrLi name | perchloric acid | lithium bromide IUPAC name | perchloric acid | lithium bromide alternate names | anhydrous perchloric acid | hydrogen perchlorate | hydrobromic acid lithium salt | lithium monobromide mass fractions | Cl (chlorine) 35.3% | H (hydrogen) 1% | O (oxygen) 63.7% | Br (bromine) 92% | Li (lithium) 7.99%
Structure diagrams
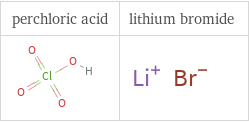
Structure diagrams
Basic properties
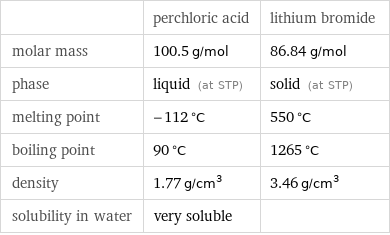
| perchloric acid | lithium bromide molar mass | 100.5 g/mol | 86.84 g/mol phase | liquid (at STP) | solid (at STP) melting point | -112 °C | 550 °C boiling point | 90 °C | 1265 °C density | 1.77 g/cm^3 | 3.46 g/cm^3 solubility in water | very soluble |
Units

Liquid properties
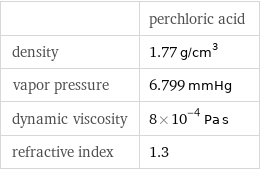
| perchloric acid density | 1.77 g/cm^3 vapor pressure | 6.799 mmHg dynamic viscosity | 8×10^-4 Pa s refractive index | 1.3
Units
Thermodynamic properties
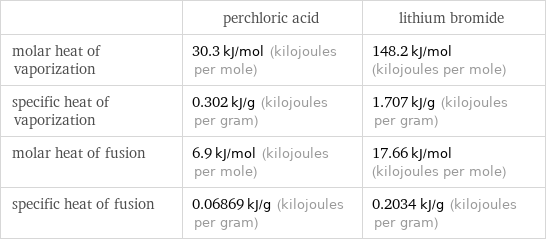
| perchloric acid | lithium bromide molar heat of vaporization | 30.3 kJ/mol (kilojoules per mole) | 148.2 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.302 kJ/g (kilojoules per gram) | 1.707 kJ/g (kilojoules per gram) molar heat of fusion | 6.9 kJ/mol (kilojoules per mole) | 17.66 kJ/mol (kilojoules per mole) specific heat of fusion | 0.06869 kJ/g (kilojoules per gram) | 0.2034 kJ/g (kilojoules per gram)
Solid properties
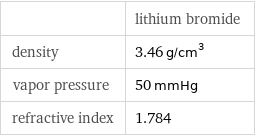
| lithium bromide density | 3.46 g/cm^3 vapor pressure | 50 mmHg refractive index | 1.784
Units
