Input interpretation

group 13 elements | atomic diameter
Summary
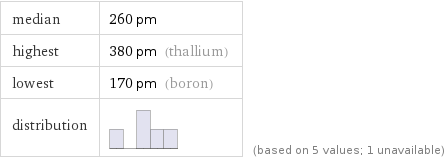
median | 260 pm highest | 380 pm (thallium) lowest | 170 pm (boron) distribution | | (based on 5 values; 1 unavailable)
Entity with missing value
nihonium
Ranked values
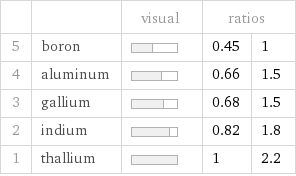
| | visual | ratios | 5 | boron | | 0.45 | 1 4 | aluminum | | 0.66 | 1.5 3 | gallium | | 0.68 | 1.5 2 | indium | | 0.82 | 1.8 1 | thallium | | 1 | 2.2
Plot
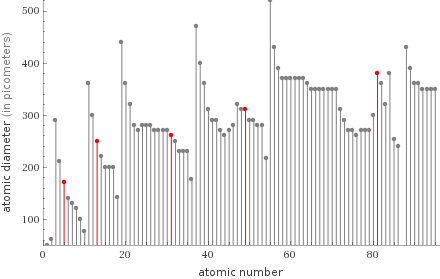
Plot
Periodic table location
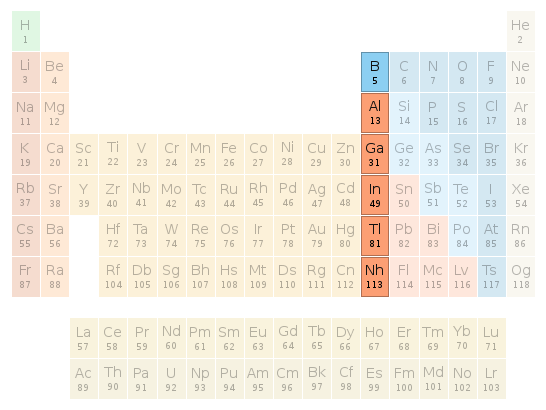
Periodic table location
Atomic radii properties
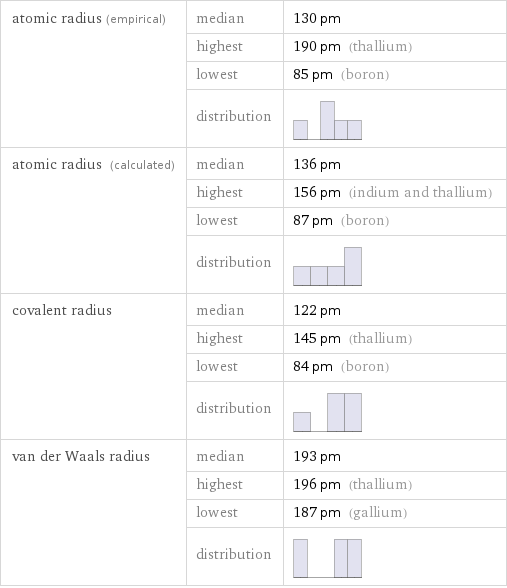
atomic radius (empirical) | median | 130 pm | highest | 190 pm (thallium) | lowest | 85 pm (boron) | distribution | atomic radius (calculated) | median | 136 pm | highest | 156 pm (indium and thallium) | lowest | 87 pm (boron) | distribution | covalent radius | median | 122 pm | highest | 145 pm (thallium) | lowest | 84 pm (boron) | distribution | van der Waals radius | median | 193 pm | highest | 196 pm (thallium) | lowest | 187 pm (gallium) | distribution |
Units

Atomic diameter rankings
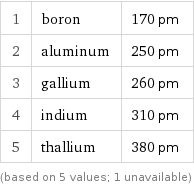
1 | boron | 170 pm 2 | aluminum | 250 pm 3 | gallium | 260 pm 4 | indium | 310 pm 5 | thallium | 380 pm (based on 5 values; 1 unavailable)
Unit conversions for median atomic diameter 260 pm

0.26 nm (nanometers)
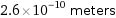
2.6×10^-10 meters
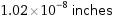
1.02×10^-8 inches
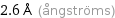
2.6 Å (ångströms)
Comparisons for median atomic diameter 260 pm
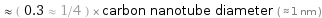
≈ ( 0.3 ≈ 1/4 ) × carbon nanotube diameter ( ≈ 1 nm )
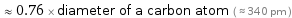
≈ 0.76 × diameter of a carbon atom ( ≈ 340 pm )
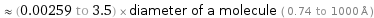
≈ (0.00259 to 3.5) × diameter of a molecule ( 0.74 to 1000 Å )