Input interpretation
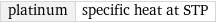
platinum | specific heat at STP
Result
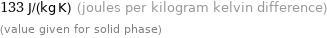
133 J/(kg K) (joules per kilogram kelvin difference) (value given for solid phase)
Unit conversions
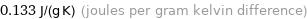
0.133 J/(g K) (joules per gram kelvin difference)
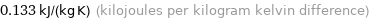
0.133 kJ/(kg K) (kilojoules per kilogram kelvin difference)
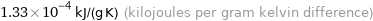
1.33×10^-4 kJ/(g K) (kilojoules per gram kelvin difference)

133 J/(kg °C) (joules per kilogram degree Celsius difference)
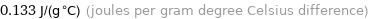
0.133 J/(g °C) (joules per gram degree Celsius difference)

0.0318 BTU_IT/(lb °F) (IT British thermal units per pound degree Fahrenheit difference)
Comparisons
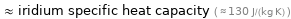
≈ iridium specific heat capacity ( ≈ 130 J/(kg K) )

≈ osmium specific heat capacity ( ≈ 130 J/(kg K) )
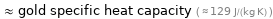
≈ gold specific heat capacity ( ≈ 129 J/(kg K) )
Thermodynamic properties
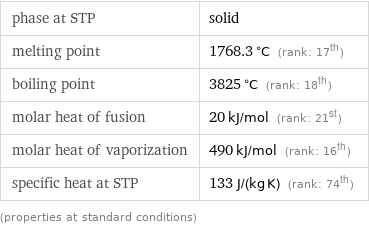
phase at STP | solid melting point | 1768.3 °C (rank: 17th) boiling point | 3825 °C (rank: 18th) molar heat of fusion | 20 kJ/mol (rank: 21st) molar heat of vaporization | 490 kJ/mol (rank: 16th) specific heat at STP | 133 J/(kg K) (rank: 74th) (properties at standard conditions)