Input interpretation
strong bases
Members
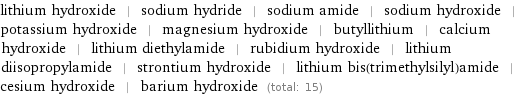
lithium hydroxide | sodium hydride | sodium amide | sodium hydroxide | potassium hydroxide | magnesium hydroxide | butyllithium | calcium hydroxide | lithium diethylamide | rubidium hydroxide | lithium diisopropylamide | strontium hydroxide | lithium bis(trimethylsilyl)amide | cesium hydroxide | barium hydroxide (total: 15)
Basic properties
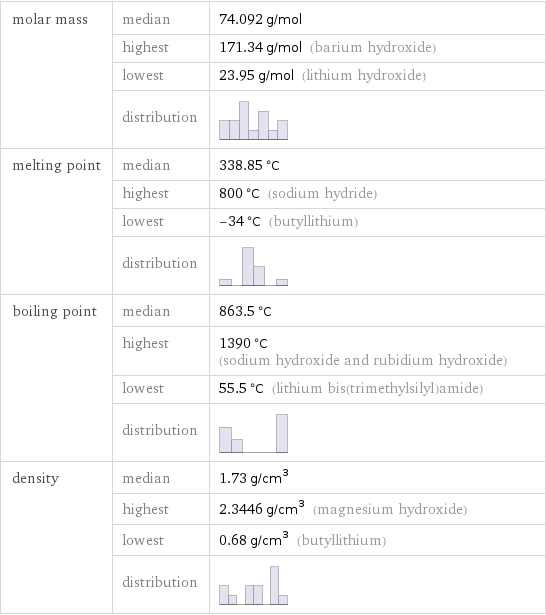
molar mass | median | 74.092 g/mol | highest | 171.34 g/mol (barium hydroxide) | lowest | 23.95 g/mol (lithium hydroxide) | distribution | melting point | median | 338.85 °C | highest | 800 °C (sodium hydride) | lowest | -34 °C (butyllithium) | distribution | boiling point | median | 863.5 °C | highest | 1390 °C (sodium hydroxide and rubidium hydroxide) | lowest | 55.5 °C (lithium bis(trimethylsilyl)amide) | distribution | density | median | 1.73 g/cm^3 | highest | 2.3446 g/cm^3 (magnesium hydroxide) | lowest | 0.68 g/cm^3 (butyllithium) | distribution |
Units
