Input interpretation
mercuric chloride | cupric cyanide
Chemical names and formulas
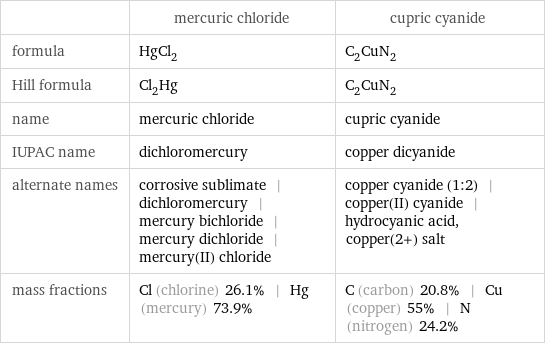
| mercuric chloride | cupric cyanide formula | HgCl_2 | C_2CuN_2 Hill formula | Cl_2Hg | C_2CuN_2 name | mercuric chloride | cupric cyanide IUPAC name | dichloromercury | copper dicyanide alternate names | corrosive sublimate | dichloromercury | mercury bichloride | mercury dichloride | mercury(II) chloride | copper cyanide (1:2) | copper(II) cyanide | hydrocyanic acid, copper(2+) salt mass fractions | Cl (chlorine) 26.1% | Hg (mercury) 73.9% | C (carbon) 20.8% | Cu (copper) 55% | N (nitrogen) 24.2%
Structure diagrams
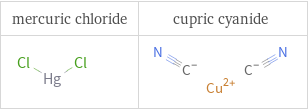
Structure diagrams
Basic properties
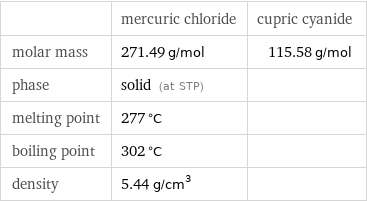
| mercuric chloride | cupric cyanide molar mass | 271.49 g/mol | 115.58 g/mol phase | solid (at STP) | melting point | 277 °C | boiling point | 302 °C | density | 5.44 g/cm^3 |
Units

Thermodynamic properties

| mercuric chloride critical temperature | 973 K (kelvins)
Solid properties
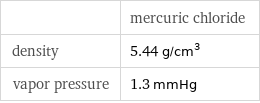
| mercuric chloride density | 5.44 g/cm^3 vapor pressure | 1.3 mmHg
Units
