Input interpretation
palladium oxide | iron(II, III) oxide
Chemical names and formulas
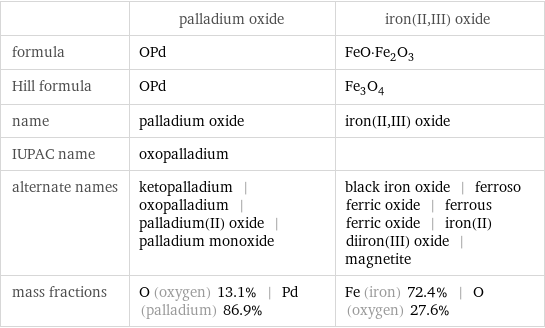
| palladium oxide | iron(II, III) oxide formula | OPd | FeO·Fe_2O_3 Hill formula | OPd | Fe_3O_4 name | palladium oxide | iron(II, III) oxide IUPAC name | oxopalladium | alternate names | ketopalladium | oxopalladium | palladium(II) oxide | palladium monoxide | black iron oxide | ferroso ferric oxide | ferrous ferric oxide | iron(II) diiron(III) oxide | magnetite mass fractions | O (oxygen) 13.1% | Pd (palladium) 86.9% | Fe (iron) 72.4% | O (oxygen) 27.6%
Structure diagrams
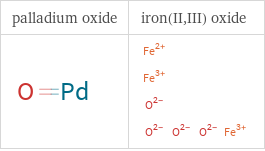
Structure diagrams
Basic properties
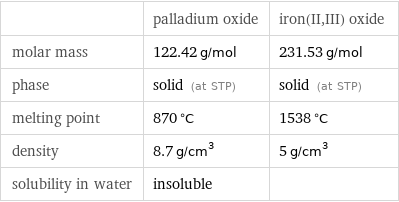
| palladium oxide | iron(II, III) oxide molar mass | 122.42 g/mol | 231.53 g/mol phase | solid (at STP) | solid (at STP) melting point | 870 °C | 1538 °C density | 8.7 g/cm^3 | 5 g/cm^3 solubility in water | insoluble |
Units
