Input interpretation
titanium (chemical element) | osmium (chemical element)
Periodic table location
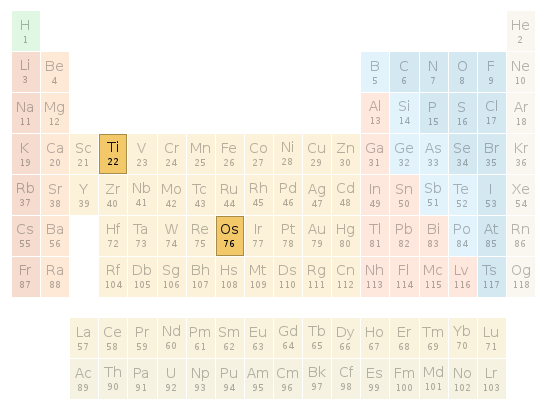
Periodic table location
Images
Images
Basic elemental properties
![| titanium | osmium atomic symbol | Ti | Os atomic number | 22 | 76 short electronic configuration | [Ar]4s^23d^2 | [Xe]6s^24f^145d^6 Aufbau diagram | 3d 4s | 5d 4f 6s block | d | d group | 4 | 8 period | 4 | 6 atomic mass | 47.867 u | 190.23 u half-life | (stable) | (stable)](../image_source/a23f8235434194035c7614a26280722a.png)
| titanium | osmium atomic symbol | Ti | Os atomic number | 22 | 76 short electronic configuration | [Ar]4s^23d^2 | [Xe]6s^24f^145d^6 Aufbau diagram | 3d 4s | 5d 4f 6s block | d | d group | 4 | 8 period | 4 | 6 atomic mass | 47.867 u | 190.23 u half-life | (stable) | (stable)
Thermodynamic properties
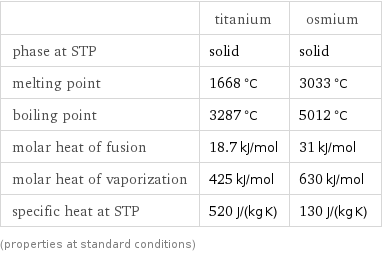
| titanium | osmium phase at STP | solid | solid melting point | 1668 °C | 3033 °C boiling point | 3287 °C | 5012 °C molar heat of fusion | 18.7 kJ/mol | 31 kJ/mol molar heat of vaporization | 425 kJ/mol | 630 kJ/mol specific heat at STP | 520 J/(kg K) | 130 J/(kg K) (properties at standard conditions)
Material properties
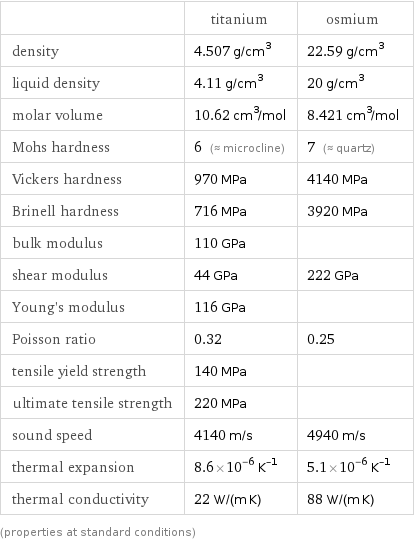
| titanium | osmium density | 4.507 g/cm^3 | 22.59 g/cm^3 liquid density | 4.11 g/cm^3 | 20 g/cm^3 molar volume | 10.62 cm^3/mol | 8.421 cm^3/mol Mohs hardness | 6 (≈ microcline) | 7 (≈ quartz) Vickers hardness | 970 MPa | 4140 MPa Brinell hardness | 716 MPa | 3920 MPa bulk modulus | 110 GPa | shear modulus | 44 GPa | 222 GPa Young's modulus | 116 GPa | Poisson ratio | 0.32 | 0.25 tensile yield strength | 140 MPa | ultimate tensile strength | 220 MPa | sound speed | 4140 m/s | 4940 m/s thermal expansion | 8.6×10^-6 K^(-1) | 5.1×10^-6 K^(-1) thermal conductivity | 22 W/(m K) | 88 W/(m K) (properties at standard conditions)
Electromagnetic properties
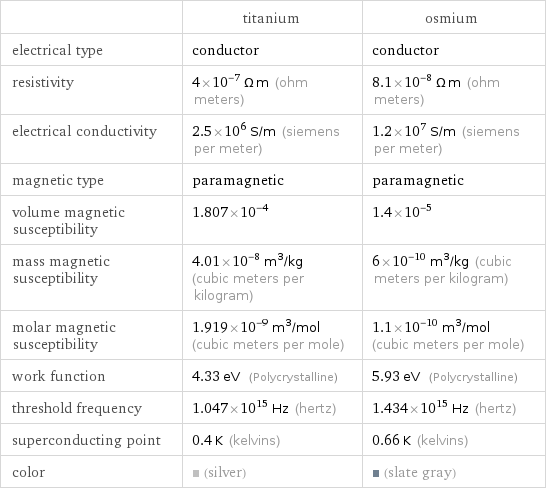
| titanium | osmium electrical type | conductor | conductor resistivity | 4×10^-7 Ω m (ohm meters) | 8.1×10^-8 Ω m (ohm meters) electrical conductivity | 2.5×10^6 S/m (siemens per meter) | 1.2×10^7 S/m (siemens per meter) magnetic type | paramagnetic | paramagnetic volume magnetic susceptibility | 1.807×10^-4 | 1.4×10^-5 mass magnetic susceptibility | 4.01×10^-8 m^3/kg (cubic meters per kilogram) | 6×10^-10 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | 1.919×10^-9 m^3/mol (cubic meters per mole) | 1.1×10^-10 m^3/mol (cubic meters per mole) work function | 4.33 eV (Polycrystalline) | 5.93 eV (Polycrystalline) threshold frequency | 1.047×10^15 Hz (hertz) | 1.434×10^15 Hz (hertz) superconducting point | 0.4 K (kelvins) | 0.66 K (kelvins) color | (silver) | (slate gray)
Reactivity
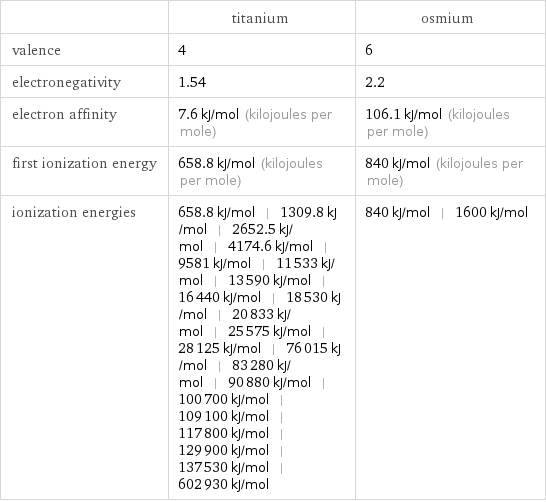
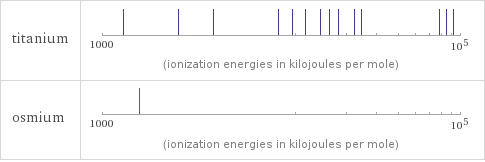
| titanium | osmium valence | 4 | 6 electronegativity | 1.54 | 2.2 electron affinity | 7.6 kJ/mol (kilojoules per mole) | 106.1 kJ/mol (kilojoules per mole) first ionization energy | 658.8 kJ/mol (kilojoules per mole) | 840 kJ/mol (kilojoules per mole) ionization energies | 658.8 kJ/mol | 1309.8 kJ/mol | 2652.5 kJ/mol | 4174.6 kJ/mol | 9581 kJ/mol | 11533 kJ/mol | 13590 kJ/mol | 16440 kJ/mol | 18530 kJ/mol | 20833 kJ/mol | 25575 kJ/mol | 28125 kJ/mol | 76015 kJ/mol | 83280 kJ/mol | 90880 kJ/mol | 100700 kJ/mol | 109100 kJ/mol | 117800 kJ/mol | 129900 kJ/mol | 137530 kJ/mol | 602930 kJ/mol | 840 kJ/mol | 1600 kJ/mol
Reactivity
Atomic properties
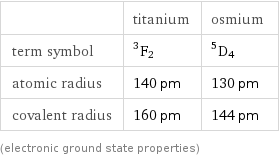
| titanium | osmium term symbol | ^3F_2 | ^5D_4 atomic radius | 140 pm | 130 pm covalent radius | 160 pm | 144 pm (electronic ground state properties)
Abundances
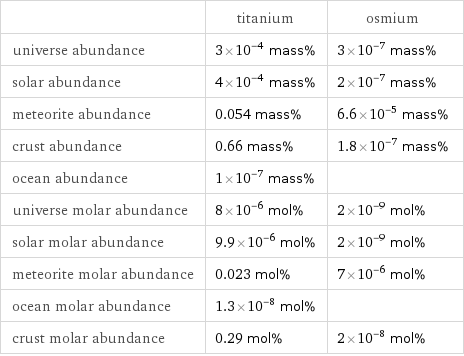
| titanium | osmium universe abundance | 3×10^-4 mass% | 3×10^-7 mass% solar abundance | 4×10^-4 mass% | 2×10^-7 mass% meteorite abundance | 0.054 mass% | 6.6×10^-5 mass% crust abundance | 0.66 mass% | 1.8×10^-7 mass% ocean abundance | 1×10^-7 mass% | universe molar abundance | 8×10^-6 mol% | 2×10^-9 mol% solar molar abundance | 9.9×10^-6 mol% | 2×10^-9 mol% meteorite molar abundance | 0.023 mol% | 7×10^-6 mol% ocean molar abundance | 1.3×10^-8 mol% | crust molar abundance | 0.29 mol% | 2×10^-8 mol%