Input interpretation
silver (chemical element) | hydrogen (chemical element) | sulfur (chemical element)
Periodic table location
Periodic table location
Images

Images
Basic elemental properties
![| silver | hydrogen | sulfur atomic symbol | Ag | H | S atomic number | 47 | 1 | 16 short electronic configuration | [Kr]5s^14d^10 | 1s^1 | [Ne]3s^23p^4 Aufbau diagram | 4d 5s | 1s | 3p 3s block | d | s | p group | 11 | 1 | 16 period | 5 | 1 | 3 atomic mass | 107.8682 u | 1.008 u | 32.06 u half-life | (stable) | (stable) | (stable)](../image_source/8706531fa806bfbd8d350a38e7fc6b70.png)
| silver | hydrogen | sulfur atomic symbol | Ag | H | S atomic number | 47 | 1 | 16 short electronic configuration | [Kr]5s^14d^10 | 1s^1 | [Ne]3s^23p^4 Aufbau diagram | 4d 5s | 1s | 3p 3s block | d | s | p group | 11 | 1 | 16 period | 5 | 1 | 3 atomic mass | 107.8682 u | 1.008 u | 32.06 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties

| silver | hydrogen | sulfur phase at STP | solid | gas | solid melting point | 961.78 °C | -259.14 °C | 115.21 °C boiling point | 2162 °C | -252.87 °C | 444.72 °C critical temperature | | 32.97 K | 1314 K critical pressure | | 1.293 MPa | 20.7 MPa molar heat of fusion | 11.3 kJ/mol | 0.558 kJ/mol | 1.73 kJ/mol molar heat of vaporization | 255 kJ/mol | 0.452 kJ/mol | 9.8 kJ/mol specific heat at STP | 235 J/(kg K) | 14300 J/(kg K) | 705 J/(kg K) adiabatic index | | 7/5 | (properties at standard conditions)
Material properties
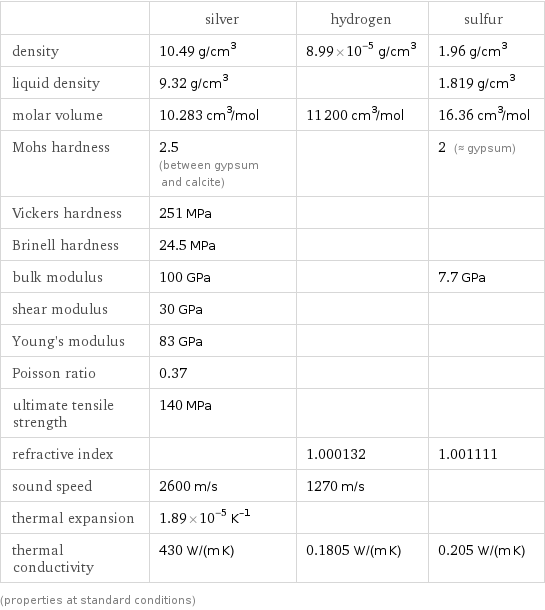
| silver | hydrogen | sulfur density | 10.49 g/cm^3 | 8.99×10^-5 g/cm^3 | 1.96 g/cm^3 liquid density | 9.32 g/cm^3 | | 1.819 g/cm^3 molar volume | 10.283 cm^3/mol | 11200 cm^3/mol | 16.36 cm^3/mol Mohs hardness | 2.5 (between gypsum and calcite) | | 2 (≈ gypsum) Vickers hardness | 251 MPa | | Brinell hardness | 24.5 MPa | | bulk modulus | 100 GPa | | 7.7 GPa shear modulus | 30 GPa | | Young's modulus | 83 GPa | | Poisson ratio | 0.37 | | ultimate tensile strength | 140 MPa | | refractive index | | 1.000132 | 1.001111 sound speed | 2600 m/s | 1270 m/s | thermal expansion | 1.89×10^-5 K^(-1) | | thermal conductivity | 430 W/(m K) | 0.1805 W/(m K) | 0.205 W/(m K) (properties at standard conditions)
Electromagnetic properties
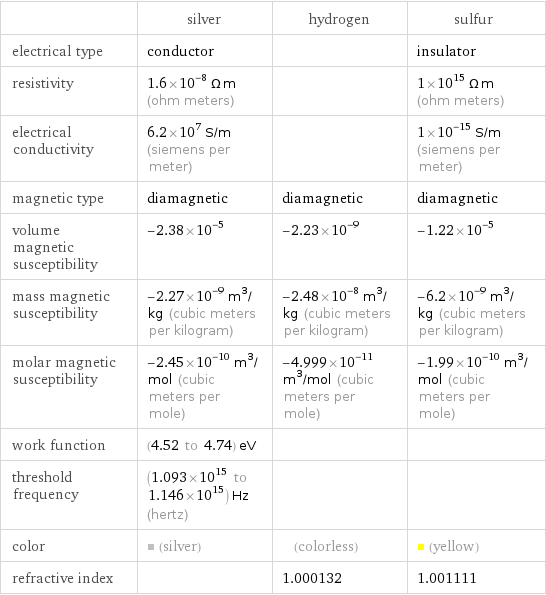
| silver | hydrogen | sulfur electrical type | conductor | | insulator resistivity | 1.6×10^-8 Ω m (ohm meters) | | 1×10^15 Ω m (ohm meters) electrical conductivity | 6.2×10^7 S/m (siemens per meter) | | 1×10^-15 S/m (siemens per meter) magnetic type | diamagnetic | diamagnetic | diamagnetic volume magnetic susceptibility | -2.38×10^-5 | -2.23×10^-9 | -1.22×10^-5 mass magnetic susceptibility | -2.27×10^-9 m^3/kg (cubic meters per kilogram) | -2.48×10^-8 m^3/kg (cubic meters per kilogram) | -6.2×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -2.45×10^-10 m^3/mol (cubic meters per mole) | -4.999×10^-11 m^3/mol (cubic meters per mole) | -1.99×10^-10 m^3/mol (cubic meters per mole) work function | (4.52 to 4.74) eV | | threshold frequency | (1.093×10^15 to 1.146×10^15) Hz (hertz) | | color | (silver) | (colorless) | (yellow) refractive index | | 1.000132 | 1.001111
Reactivity
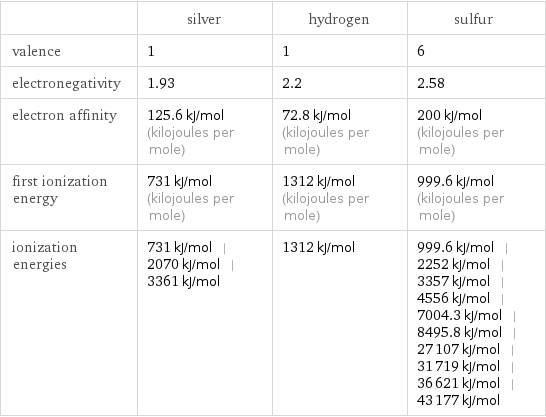
| silver | hydrogen | sulfur valence | 1 | 1 | 6 electronegativity | 1.93 | 2.2 | 2.58 electron affinity | 125.6 kJ/mol (kilojoules per mole) | 72.8 kJ/mol (kilojoules per mole) | 200 kJ/mol (kilojoules per mole) first ionization energy | 731 kJ/mol (kilojoules per mole) | 1312 kJ/mol (kilojoules per mole) | 999.6 kJ/mol (kilojoules per mole) ionization energies | 731 kJ/mol | 2070 kJ/mol | 3361 kJ/mol | 1312 kJ/mol | 999.6 kJ/mol | 2252 kJ/mol | 3357 kJ/mol | 4556 kJ/mol | 7004.3 kJ/mol | 8495.8 kJ/mol | 27107 kJ/mol | 31719 kJ/mol | 36621 kJ/mol | 43177 kJ/mol
Reactivity
Atomic properties
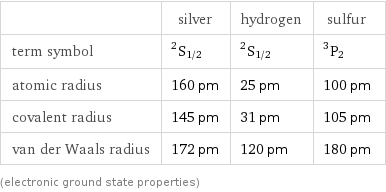
| silver | hydrogen | sulfur term symbol | ^2S_(1/2) | ^2S_(1/2) | ^3P_2 atomic radius | 160 pm | 25 pm | 100 pm covalent radius | 145 pm | 31 pm | 105 pm van der Waals radius | 172 pm | 120 pm | 180 pm (electronic ground state properties)
Abundances
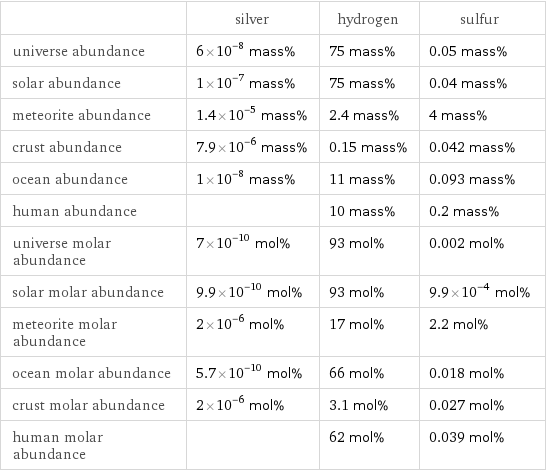
| silver | hydrogen | sulfur universe abundance | 6×10^-8 mass% | 75 mass% | 0.05 mass% solar abundance | 1×10^-7 mass% | 75 mass% | 0.04 mass% meteorite abundance | 1.4×10^-5 mass% | 2.4 mass% | 4 mass% crust abundance | 7.9×10^-6 mass% | 0.15 mass% | 0.042 mass% ocean abundance | 1×10^-8 mass% | 11 mass% | 0.093 mass% human abundance | | 10 mass% | 0.2 mass% universe molar abundance | 7×10^-10 mol% | 93 mol% | 0.002 mol% solar molar abundance | 9.9×10^-10 mol% | 93 mol% | 9.9×10^-4 mol% meteorite molar abundance | 2×10^-6 mol% | 17 mol% | 2.2 mol% ocean molar abundance | 5.7×10^-10 mol% | 66 mol% | 0.018 mol% crust molar abundance | 2×10^-6 mol% | 3.1 mol% | 0.027 mol% human molar abundance | | 62 mol% | 0.039 mol%