Input interpretation
metal carbonyls | molar mass
Summary

median | 351.9 g/mol highest | 1105 g/mol (tetrairidium dodecacarbonyl) lowest | 141.09 g/mol (tetramethylammonium(1-hydroxyethylidene)pentacarbonylchromium) distribution | | (based on 69 values; 1 unavailable)
Entity with missing value
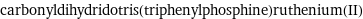
carbonyldihydridotris(triphenylphosphine)ruthenium(II)
Distribution plots
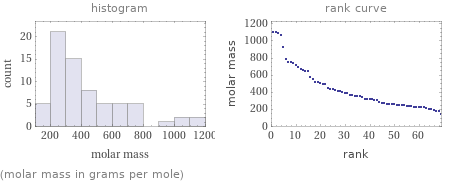
(molar mass in grams per mole)
Molar mass rankings
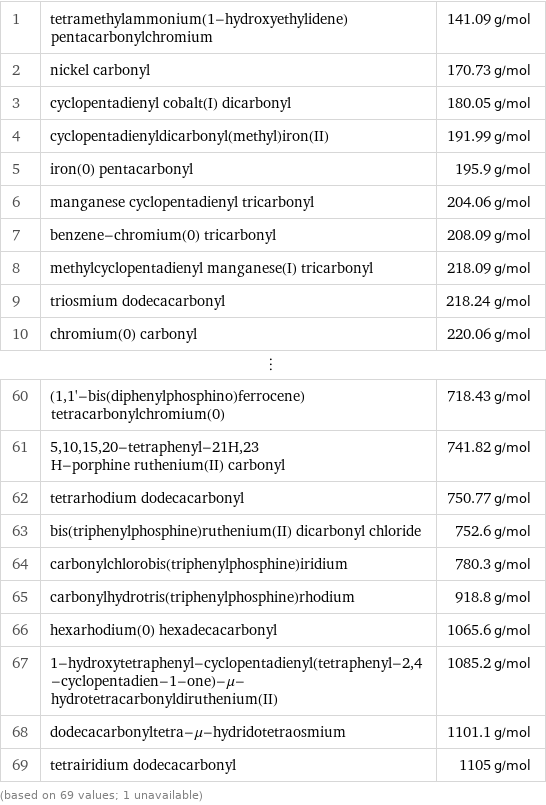
1 | tetramethylammonium(1-hydroxyethylidene)pentacarbonylchromium | 141.09 g/mol 2 | nickel carbonyl | 170.73 g/mol 3 | cyclopentadienyl cobalt(I) dicarbonyl | 180.05 g/mol 4 | cyclopentadienyldicarbonyl(methyl)iron(II) | 191.99 g/mol 5 | iron(0) pentacarbonyl | 195.9 g/mol 6 | manganese cyclopentadienyl tricarbonyl | 204.06 g/mol 7 | benzene-chromium(0) tricarbonyl | 208.09 g/mol 8 | methylcyclopentadienyl manganese(I) tricarbonyl | 218.09 g/mol 9 | triosmium dodecacarbonyl | 218.24 g/mol 10 | chromium(0) carbonyl | 220.06 g/mol ⋮ | | 60 | (1, 1'-bis(diphenylphosphino)ferrocene)tetracarbonylchromium(0) | 718.43 g/mol 61 | 5, 10, 15, 20-tetraphenyl-21H, 23 H-porphine ruthenium(II) carbonyl | 741.82 g/mol 62 | tetrarhodium dodecacarbonyl | 750.77 g/mol 63 | bis(triphenylphosphine)ruthenium(II) dicarbonyl chloride | 752.6 g/mol 64 | carbonylchlorobis(triphenylphosphine)iridium | 780.3 g/mol 65 | carbonylhydrotris(triphenylphosphine)rhodium | 918.8 g/mol 66 | hexarhodium(0) hexadecacarbonyl | 1065.6 g/mol 67 | 1-hydroxytetraphenyl-cyclopentadienyl(tetraphenyl-2, 4-cyclopentadien-1-one)-μ-hydrotetracarbonyldiruthenium(II) | 1085.2 g/mol 68 | dodecacarbonyltetra-μ-hydridotetraosmium | 1101.1 g/mol 69 | tetrairidium dodecacarbonyl | 1105 g/mol (based on 69 values; 1 unavailable)
Unit conversion for median molar mass 351.9 g/mol
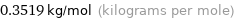
0.3519 kg/mol (kilograms per mole)
Comparisons for median molar mass 351.9 g/mol
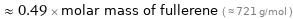
≈ 0.49 × molar mass of fullerene ( ≈ 721 g/mol )
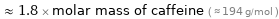
≈ 1.8 × molar mass of caffeine ( ≈ 194 g/mol )
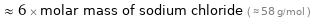
≈ 6 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
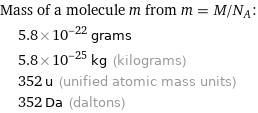
Mass of a molecule m from m = M/N_A: | 5.8×10^-22 grams | 5.8×10^-25 kg (kilograms) | 352 u (unified atomic mass units) | 352 Da (daltons)
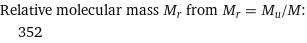
Relative molecular mass M_r from M_r = M_u/M: | 352