Input interpretation
mercuric sulfate | cesium hydroxide monohydrate
Chemical names and formulas
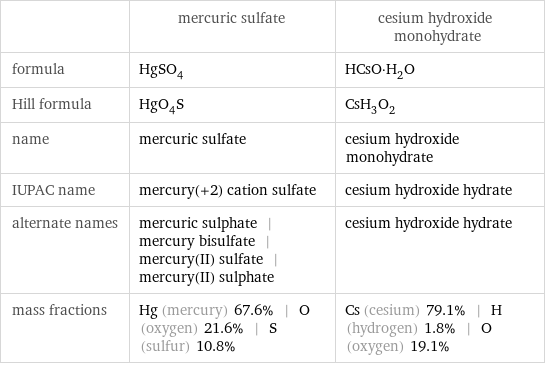
| mercuric sulfate | cesium hydroxide monohydrate formula | HgSO_4 | HCsO·H_2O Hill formula | HgO_4S | CsH_3O_2 name | mercuric sulfate | cesium hydroxide monohydrate IUPAC name | mercury(+2) cation sulfate | cesium hydroxide hydrate alternate names | mercuric sulphate | mercury bisulfate | mercury(II) sulfate | mercury(II) sulphate | cesium hydroxide hydrate mass fractions | Hg (mercury) 67.6% | O (oxygen) 21.6% | S (sulfur) 10.8% | Cs (cesium) 79.1% | H (hydrogen) 1.8% | O (oxygen) 19.1%
Structure diagrams
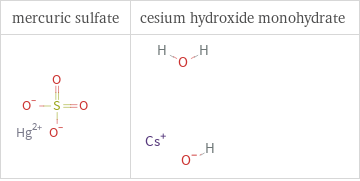
Structure diagrams
Basic properties
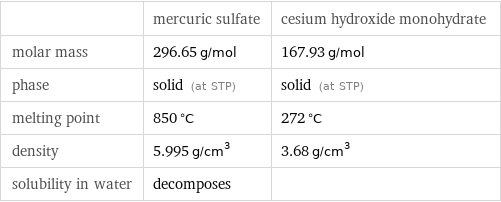
| mercuric sulfate | cesium hydroxide monohydrate molar mass | 296.65 g/mol | 167.93 g/mol phase | solid (at STP) | solid (at STP) melting point | 850 °C | 272 °C density | 5.995 g/cm^3 | 3.68 g/cm^3 solubility in water | decomposes |
Units

Thermodynamic properties
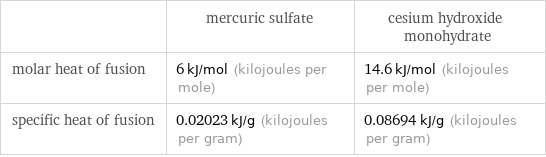
| mercuric sulfate | cesium hydroxide monohydrate molar heat of fusion | 6 kJ/mol (kilojoules per mole) | 14.6 kJ/mol (kilojoules per mole) specific heat of fusion | 0.02023 kJ/g (kilojoules per gram) | 0.08694 kJ/g (kilojoules per gram)