Input interpretation
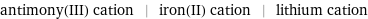
antimony(III) cation | iron(II) cation | lithium cation
Structure diagrams
Structure diagrams
General properties
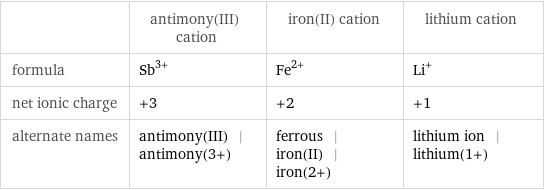
| antimony(III) cation | iron(II) cation | lithium cation formula | Sb^(3+) | Fe^(2+) | Li^+ net ionic charge | +3 | +2 | +1 alternate names | antimony(III) | antimony(3+) | ferrous | iron(II) | iron(2+) | lithium ion | lithium(1+)
Ionic radii
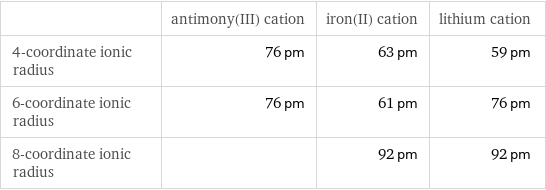
| antimony(III) cation | iron(II) cation | lithium cation 4-coordinate ionic radius | 76 pm | 63 pm | 59 pm 6-coordinate ionic radius | 76 pm | 61 pm | 76 pm 8-coordinate ionic radius | | 92 pm | 92 pm
Units

Other properties
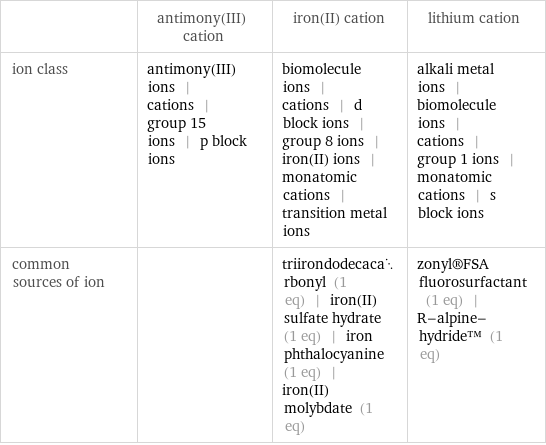
| antimony(III) cation | iron(II) cation | lithium cation ion class | antimony(III) ions | cations | group 15 ions | p block ions | biomolecule ions | cations | d block ions | group 8 ions | iron(II) ions | monatomic cations | transition metal ions | alkali metal ions | biomolecule ions | cations | group 1 ions | monatomic cations | s block ions common sources of ion | | triirondodecacarbonyl (1 eq) | iron(II) sulfate hydrate (1 eq) | iron phthalocyanine (1 eq) | iron(II) molybdate (1 eq) | zonyl®FSA fluorosurfactant (1 eq) | R-alpine-hydride™ (1 eq)