Input interpretation
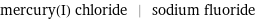
mercury(I) chloride | sodium fluoride
Chemical names and formulas
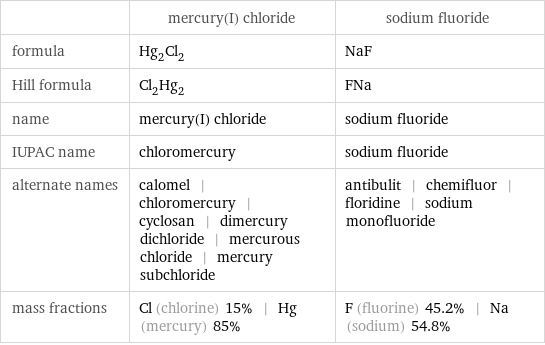
| mercury(I) chloride | sodium fluoride formula | Hg_2Cl_2 | NaF Hill formula | Cl_2Hg_2 | FNa name | mercury(I) chloride | sodium fluoride IUPAC name | chloromercury | sodium fluoride alternate names | calomel | chloromercury | cyclosan | dimercury dichloride | mercurous chloride | mercury subchloride | antibulit | chemifluor | floridine | sodium monofluoride mass fractions | Cl (chlorine) 15% | Hg (mercury) 85% | F (fluorine) 45.2% | Na (sodium) 54.8%
Structure diagrams
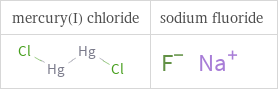
Structure diagrams
Basic properties
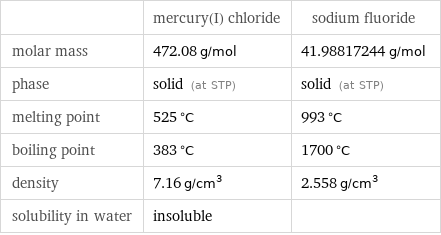
| mercury(I) chloride | sodium fluoride molar mass | 472.08 g/mol | 41.98817244 g/mol phase | solid (at STP) | solid (at STP) melting point | 525 °C | 993 °C boiling point | 383 °C | 1700 °C density | 7.16 g/cm^3 | 2.558 g/cm^3 solubility in water | insoluble |
Units

Thermodynamic properties

| mercury(I) chloride | sodium fluoride molar heat of fusion | 15.1 kJ/mol (kilojoules per mole) | 33.35 kJ/mol (kilojoules per mole) specific heat of fusion | 0.03199 kJ/g (kilojoules per gram) | 0.7943 kJ/g (kilojoules per gram)
Solid properties (at STP)

| mercury(I) chloride | sodium fluoride density | 7.16 g/cm^3 | 2.558 g/cm^3 vapor pressure | 1×10^-4 mmHg | 1.4 mmHg
Units

Chemical identifiers
![| mercury(I) chloride | sodium fluoride CAS number | 10112-91-1 | 7681-49-4 PubChem CID number | 24956 | 5235 PubChem SID number | 24868461 | 24852884 SMILES identifier | Cl[Hg].Cl[Hg] | [F-].[Na+]](../image_source/0af0c2d4dbc4eb899d57dfcc1dfd052a.png)
| mercury(I) chloride | sodium fluoride CAS number | 10112-91-1 | 7681-49-4 PubChem CID number | 24956 | 5235 PubChem SID number | 24868461 | 24852884 SMILES identifier | Cl[Hg].Cl[Hg] | [F-].[Na+]