Input interpretation
sodium
Chemical names and formulas
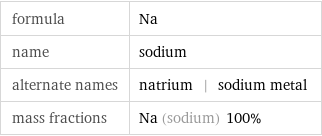
formula | Na name | sodium alternate names | natrium | sodium metal mass fractions | Na (sodium) 100%
Structure diagram

Structure diagram
Basic properties
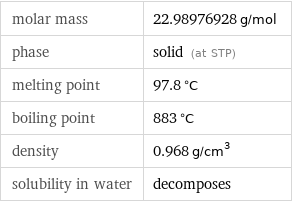
molar mass | 22.98976928 g/mol phase | solid (at STP) melting point | 97.8 °C boiling point | 883 °C density | 0.968 g/cm^3 solubility in water | decomposes
Units

Solid properties (at STP)
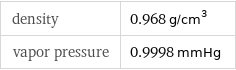
density | 0.968 g/cm^3 vapor pressure | 0.9998 mmHg
Units

Thermodynamic properties
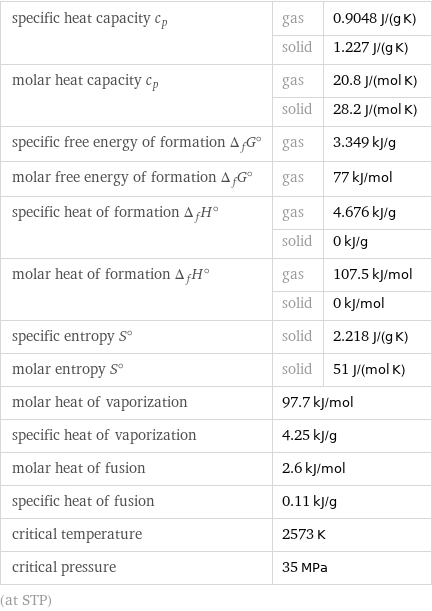
specific heat capacity c_p | gas | 0.9048 J/(g K) | solid | 1.227 J/(g K) molar heat capacity c_p | gas | 20.8 J/(mol K) | solid | 28.2 J/(mol K) specific free energy of formation Δ_fG° | gas | 3.349 kJ/g molar free energy of formation Δ_fG° | gas | 77 kJ/mol specific heat of formation Δ_fH° | gas | 4.676 kJ/g | solid | 0 kJ/g molar heat of formation Δ_fH° | gas | 107.5 kJ/mol | solid | 0 kJ/mol specific entropy S° | solid | 2.218 J/(g K) molar entropy S° | solid | 51 J/(mol K) molar heat of vaporization | 97.7 kJ/mol | specific heat of vaporization | 4.25 kJ/g | molar heat of fusion | 2.6 kJ/mol | specific heat of fusion | 0.11 kJ/g | critical temperature | 2573 K | critical pressure | 35 MPa | (at STP)
Chemical identifiers
![CAS number | 7440-23-5 PubChem CID number | 5360545 PubChem SID number | 24855921 SMILES identifier | [Na] InChI identifier | InChI=1/Na RTECS number | VY0686000 MDL number | MFCD00085307](../image_source/a915dce0a2d9ad21e0a919f0f7bbacd7.png)
CAS number | 7440-23-5 PubChem CID number | 5360545 PubChem SID number | 24855921 SMILES identifier | [Na] InChI identifier | InChI=1/Na RTECS number | VY0686000 MDL number | MFCD00085307
NFPA label

NFPA label
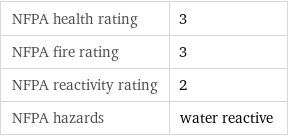
NFPA health rating | 3 NFPA fire rating | 3 NFPA reactivity rating | 2 NFPA hazards | water reactive
Safety properties

flash point | 4 °C autoignition point | 115 °C

DOT hazard class | 4.3 DOT numbers | 1428
Toxicity properties

RTECS classes | other