Input interpretation
p block ions | total number of electrons
Distribution plots
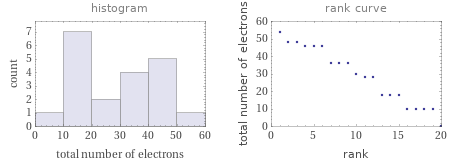
Total number of electrons rankings
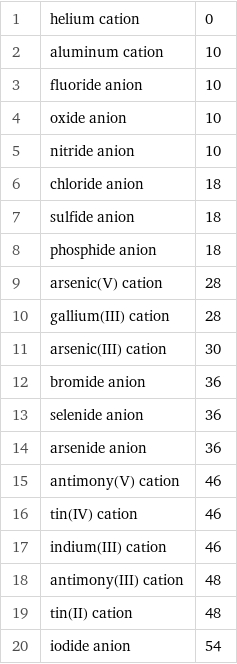
1 | helium cation | 0 2 | aluminum cation | 10 3 | fluoride anion | 10 4 | oxide anion | 10 5 | nitride anion | 10 6 | chloride anion | 18 7 | sulfide anion | 18 8 | phosphide anion | 18 9 | arsenic(V) cation | 28 10 | gallium(III) cation | 28 11 | arsenic(III) cation | 30 12 | bromide anion | 36 13 | selenide anion | 36 14 | arsenide anion | 36 15 | antimony(V) cation | 46 16 | tin(IV) cation | 46 17 | indium(III) cation | 46 18 | antimony(III) cation | 48 19 | tin(II) cation | 48 20 | iodide anion | 54