Input interpretation
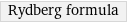
Rydberg formula
Equation
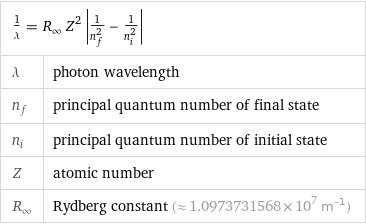
1/λ = R_∞ Z^2 abs(1/n_f^2 - 1/n_i^2) | λ | photon wavelength n_f | principal quantum number of final state n_i | principal quantum number of initial state Z | atomic number R_∞ | Rydberg constant (≈ 1.0973731568×10^7 m^(-1))
Input values
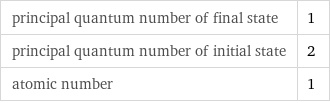
principal quantum number of final state | 1 principal quantum number of initial state | 2 atomic number | 1
Results

photon wavelength | 121.5 nm (nanometers) = 1.215×10^-7 meters photon frequency | 2.467×10^15 Hz (hertz) photon energy | 1.635×10^-18 J (joules) photon color | (ultraviolet) series of transition | Lyman