Input interpretation

hydrobromofluorocarbons | molar mass
Summary

median | 225.01 g/mol highest | 337.89 g/mol (1, 5-dibromo-1, 1, 3, 3, 5, 5-hexafluoropentane) lowest | 126.96 g/mol (1-bromo-2-fluoroethane) distribution |
Units

Distribution plots
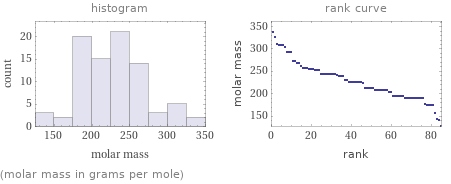
(molar mass in grams per mole)
Molar mass rankings
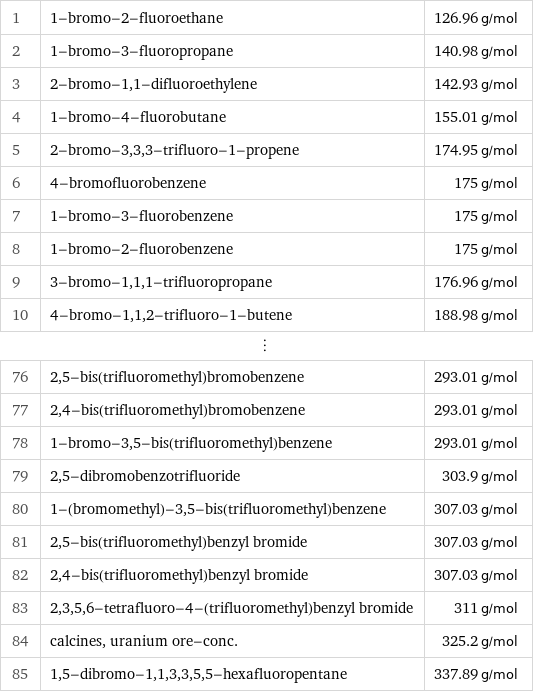
1 | 1-bromo-2-fluoroethane | 126.96 g/mol 2 | 1-bromo-3-fluoropropane | 140.98 g/mol 3 | 2-bromo-1, 1-difluoroethylene | 142.93 g/mol 4 | 1-bromo-4-fluorobutane | 155.01 g/mol 5 | 2-bromo-3, 3, 3-trifluoro-1-propene | 174.95 g/mol 6 | 4-bromofluorobenzene | 175 g/mol 7 | 1-bromo-3-fluorobenzene | 175 g/mol 8 | 1-bromo-2-fluorobenzene | 175 g/mol 9 | 3-bromo-1, 1, 1-trifluoropropane | 176.96 g/mol 10 | 4-bromo-1, 1, 2-trifluoro-1-butene | 188.98 g/mol ⋮ | | 76 | 2, 5-bis(trifluoromethyl)bromobenzene | 293.01 g/mol 77 | 2, 4-bis(trifluoromethyl)bromobenzene | 293.01 g/mol 78 | 1-bromo-3, 5-bis(trifluoromethyl)benzene | 293.01 g/mol 79 | 2, 5-dibromobenzotrifluoride | 303.9 g/mol 80 | 1-(bromomethyl)-3, 5-bis(trifluoromethyl)benzene | 307.03 g/mol 81 | 2, 5-bis(trifluoromethyl)benzyl bromide | 307.03 g/mol 82 | 2, 4-bis(trifluoromethyl)benzyl bromide | 307.03 g/mol 83 | 2, 3, 5, 6-tetrafluoro-4-(trifluoromethyl)benzyl bromide | 311 g/mol 84 | calcines, uranium ore-conc. | 325.2 g/mol 85 | 1, 5-dibromo-1, 1, 3, 3, 5, 5-hexafluoropentane | 337.89 g/mol
Unit conversion for median molar mass 225.01 g/mol
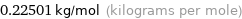
0.22501 kg/mol (kilograms per mole)
Comparisons for median molar mass 225.01 g/mol
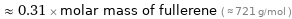
≈ 0.31 × molar mass of fullerene ( ≈ 721 g/mol )
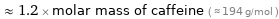
≈ 1.2 × molar mass of caffeine ( ≈ 194 g/mol )
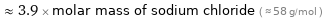
≈ 3.9 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
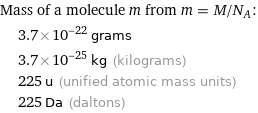
Mass of a molecule m from m = M/N_A: | 3.7×10^-22 grams | 3.7×10^-25 kg (kilograms) | 225 u (unified atomic mass units) | 225 Da (daltons)
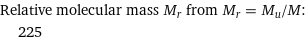
Relative molecular mass M_r from M_r = M_u/M: | 225