Input interpretation

Henderson-Hasselbalch equation
Equation
![pH = pK_a + log_10([B]/[HA]) | [B] | base concentration pH | pH pK_a | log acidity constant [HA] | acid concentration (assuming concentrations are in moles/liter)](../image_source/6759dab58f5944fa479317762be81f66.png)
pH = pK_a + log_10([B]/[HA]) | [B] | base concentration pH | pH pK_a | log acidity constant [HA] | acid concentration (assuming concentrations are in moles/liter)
Input values
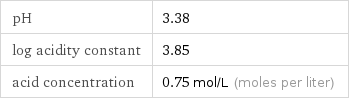
pH | 3.38 log acidity constant | 3.85 acid concentration | 0.75 mol/L (moles per liter)
Results

base concentration | 254.1 mmol/L (millimoles per liter) = 0.2541 mol/L (moles per liter) = 254.1 mM (millimolar) = 0.2541 M (molar) = 254.1 mol/m^3 (moles per cubic meter) = 0.2541 mol/dm^3 (moles per cubic decimeter) = 25.41 mmol/dL (millimoles per deciliter) = 254.1 µmol/mL (micromoles per milliliter) = 0.2541 mmol/mL (millimoles per milliliter) = 0.02541 mol/dL (moles per deciliter)
Possible intermediate steps
![Calculate the base concentration using the following information: known variables | | pH | pH | 3.38 pK_a | log acidity constant | 3.85 [HA] | acid concentration | 0.75 mol/L The relevant equation that relates base concentration ([B]), pH (pH), log acidity constant (pK_a), and acid concentration ([HA]) is: pH = pK_a + log_10([B]/[HA]) pH = pK_a + log([B]/[HA])/log(10) is equivalent to pK_a + log([B]/[HA])/log(10) = pH: pK_a + log([B]/[HA])/log(10) = pH Subtract pK_a from both sides: log([B]/[HA])/log(10) = pH - pK_a Multiply both sides by log(10): log([B]/[HA]) = pH log(10) - pK_a log(10) Cancel logarithms by taking exp of both sides: [B]/[HA] = e^(pH log(10) - pK_a log(10)) Multiply both sides by [HA]: [B] = e^(pH log(10) - pK_a log(10)) [HA] Substitute known variables into the equation: known variables | | pH | pH | 3.38 pK_a | log acidity constant | 3.85 [HA] | acid concentration | 0.75 mol/L | : [B] = 0.75 mol/L e^(3.38 log(10) - 3.85 log(10)) Separate the numerical part, 0.75 e^(3.38 log(10) - 3.85 log(10)), from the unit part, mol/L: [B] = 0.75 e^(3.38 log(10) - 3.85 log(10)) mol/L Evaluate 0.75 e^(3.38 log(10) - 3.85 log(10)): [B] = 0.25413 mol/L Convert 0.25413 mol/L into mmol/L (millimoles per liter) using the following: 1 mol/L = 1000 mmol/L: Answer: | | [B] = 254.1 mmol/L](../image_source/73507e04854585c3944d71ba315b08d7.png)
Calculate the base concentration using the following information: known variables | | pH | pH | 3.38 pK_a | log acidity constant | 3.85 [HA] | acid concentration | 0.75 mol/L The relevant equation that relates base concentration ([B]), pH (pH), log acidity constant (pK_a), and acid concentration ([HA]) is: pH = pK_a + log_10([B]/[HA]) pH = pK_a + log([B]/[HA])/log(10) is equivalent to pK_a + log([B]/[HA])/log(10) = pH: pK_a + log([B]/[HA])/log(10) = pH Subtract pK_a from both sides: log([B]/[HA])/log(10) = pH - pK_a Multiply both sides by log(10): log([B]/[HA]) = pH log(10) - pK_a log(10) Cancel logarithms by taking exp of both sides: [B]/[HA] = e^(pH log(10) - pK_a log(10)) Multiply both sides by [HA]: [B] = e^(pH log(10) - pK_a log(10)) [HA] Substitute known variables into the equation: known variables | | pH | pH | 3.38 pK_a | log acidity constant | 3.85 [HA] | acid concentration | 0.75 mol/L | : [B] = 0.75 mol/L e^(3.38 log(10) - 3.85 log(10)) Separate the numerical part, 0.75 e^(3.38 log(10) - 3.85 log(10)), from the unit part, mol/L: [B] = 0.75 e^(3.38 log(10) - 3.85 log(10)) mol/L Evaluate 0.75 e^(3.38 log(10) - 3.85 log(10)): [B] = 0.25413 mol/L Convert 0.25413 mol/L into mmol/L (millimoles per liter) using the following: 1 mol/L = 1000 mmol/L: Answer: | | [B] = 254.1 mmol/L