Input interpretation
iodine | carbon monoxide
Chemical names and formulas
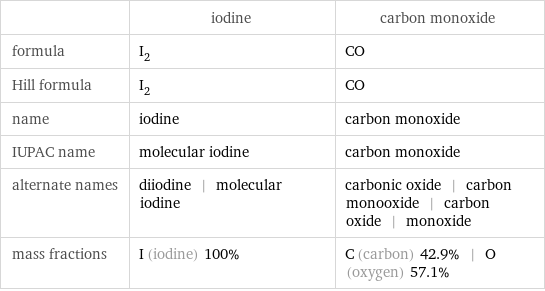
| iodine | carbon monoxide formula | I_2 | CO Hill formula | I_2 | CO name | iodine | carbon monoxide IUPAC name | molecular iodine | carbon monoxide alternate names | diiodine | molecular iodine | carbonic oxide | carbon monooxide | carbon oxide | monoxide mass fractions | I (iodine) 100% | C (carbon) 42.9% | O (oxygen) 57.1%
Structure diagrams
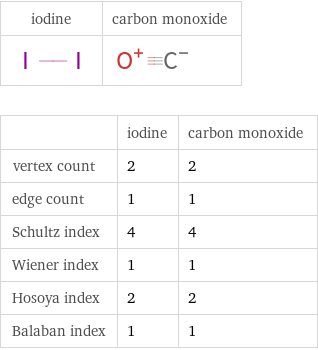
| iodine | carbon monoxide vertex count | 2 | 2 edge count | 1 | 1 Schultz index | 4 | 4 Wiener index | 1 | 1 Hosoya index | 2 | 2 Balaban index | 1 | 1
3D structure

3D structure
Basic properties

| iodine | carbon monoxide molar mass | 253.80894 g/mol | 28.01 g/mol phase | solid (at STP) | gas (at STP) melting point | 113 °C | -205 °C boiling point | 184 °C | -191.5 °C density | 4.94 g/cm^3 | 0.001145 g/cm^3
Units

Gas properties
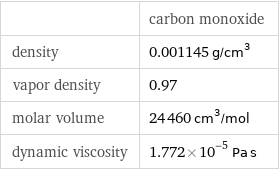
| carbon monoxide density | 0.001145 g/cm^3 vapor density | 0.97 molar volume | 24460 cm^3/mol dynamic viscosity | 1.772×10^-5 Pa s
Units
