Input interpretation

top 10 chemicals | by molar heat of fusion
Result
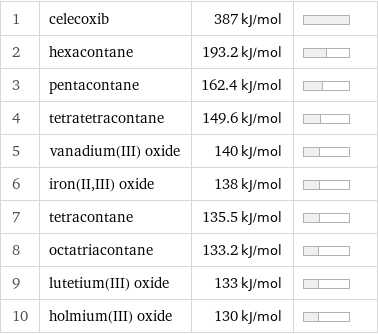
1 | celecoxib | 387 kJ/mol | 2 | hexacontane | 193.2 kJ/mol | 3 | pentacontane | 162.4 kJ/mol | 4 | tetratetracontane | 149.6 kJ/mol | 5 | vanadium(III) oxide | 140 kJ/mol | 6 | iron(II, III) oxide | 138 kJ/mol | 7 | tetracontane | 135.5 kJ/mol | 8 | octatriacontane | 133.2 kJ/mol | 9 | lutetium(III) oxide | 133 kJ/mol | 10 | holmium(III) oxide | 130 kJ/mol |
Structure diagrams
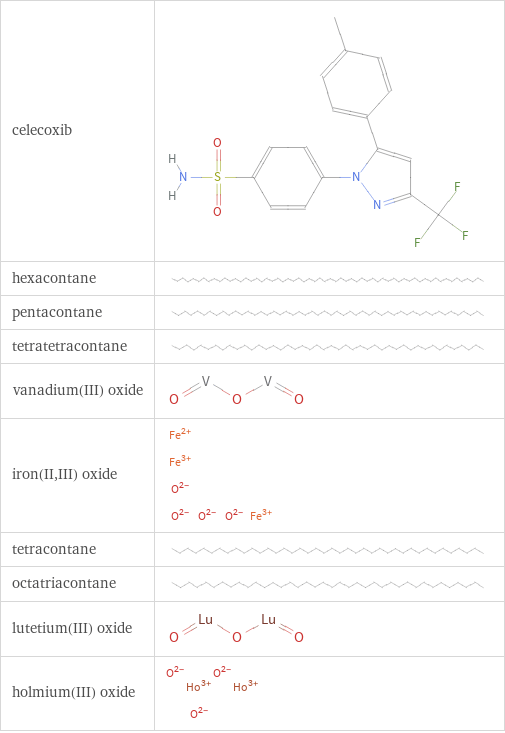
Structure diagrams
Basic properties
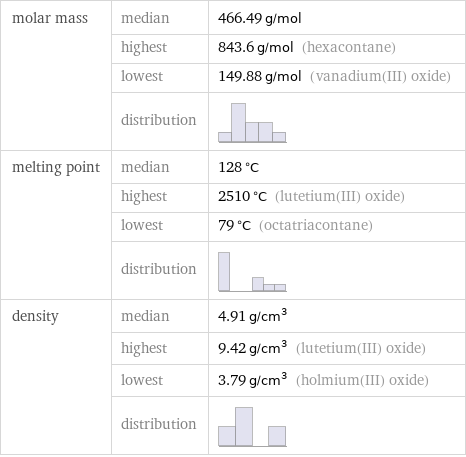
molar mass | median | 466.49 g/mol | highest | 843.6 g/mol (hexacontane) | lowest | 149.88 g/mol (vanadium(III) oxide) | distribution | melting point | median | 128 °C | highest | 2510 °C (lutetium(III) oxide) | lowest | 79 °C (octatriacontane) | distribution | density | median | 4.91 g/cm^3 | highest | 9.42 g/cm^3 (lutetium(III) oxide) | lowest | 3.79 g/cm^3 (holmium(III) oxide) | distribution |
Units
