Input interpretation
titanyl cation | ion class
Result
cations | oxocations | titanium(IV) ions
Ionic radii
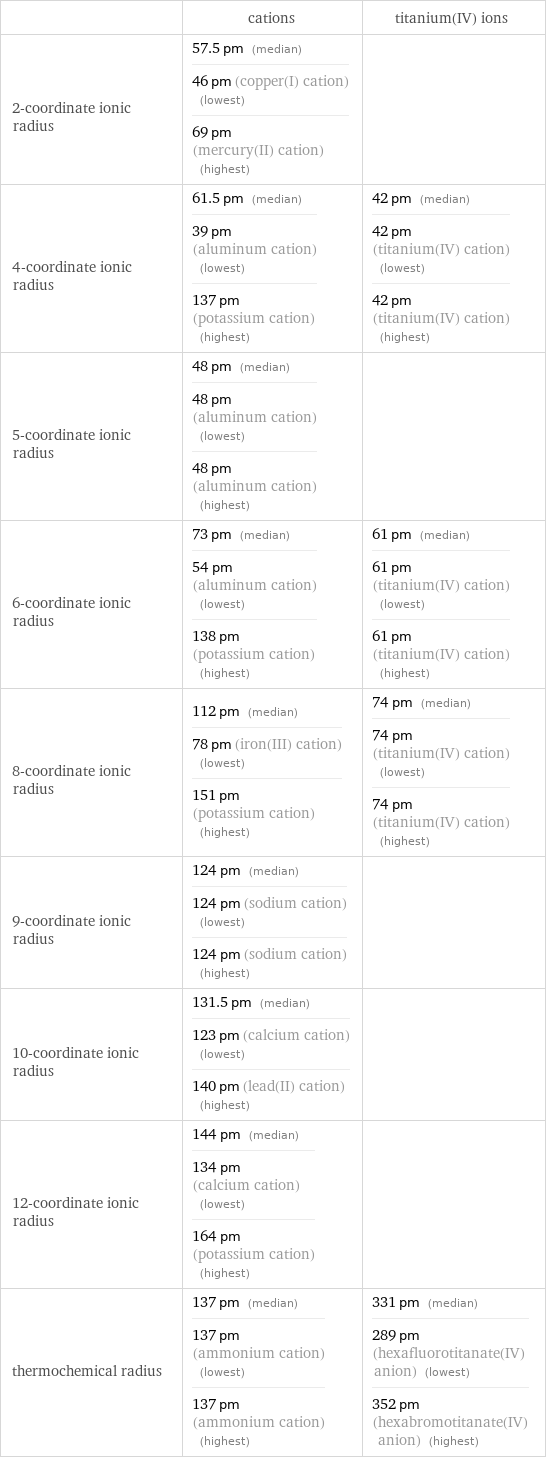
| cations | titanium(IV) ions 2-coordinate ionic radius | 57.5 pm (median) 46 pm (copper(I) cation) (lowest) 69 pm (mercury(II) cation) (highest) | 4-coordinate ionic radius | 61.5 pm (median) 39 pm (aluminum cation) (lowest) 137 pm (potassium cation) (highest) | 42 pm (median) 42 pm (titanium(IV) cation) (lowest) 42 pm (titanium(IV) cation) (highest) 5-coordinate ionic radius | 48 pm (median) 48 pm (aluminum cation) (lowest) 48 pm (aluminum cation) (highest) | 6-coordinate ionic radius | 73 pm (median) 54 pm (aluminum cation) (lowest) 138 pm (potassium cation) (highest) | 61 pm (median) 61 pm (titanium(IV) cation) (lowest) 61 pm (titanium(IV) cation) (highest) 8-coordinate ionic radius | 112 pm (median) 78 pm (iron(III) cation) (lowest) 151 pm (potassium cation) (highest) | 74 pm (median) 74 pm (titanium(IV) cation) (lowest) 74 pm (titanium(IV) cation) (highest) 9-coordinate ionic radius | 124 pm (median) 124 pm (sodium cation) (lowest) 124 pm (sodium cation) (highest) | 10-coordinate ionic radius | 131.5 pm (median) 123 pm (calcium cation) (lowest) 140 pm (lead(II) cation) (highest) | 12-coordinate ionic radius | 144 pm (median) 134 pm (calcium cation) (lowest) 164 pm (potassium cation) (highest) | thermochemical radius | 137 pm (median) 137 pm (ammonium cation) (lowest) 137 pm (ammonium cation) (highest) | 331 pm (median) 289 pm (hexafluorotitanate(IV) anion) (lowest) 352 pm (hexabromotitanate(IV) anion) (highest)