Input interpretation

alkali metals | van der Waals radius
Summary
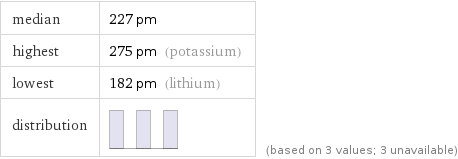
median | 227 pm highest | 275 pm (potassium) lowest | 182 pm (lithium) distribution | | (based on 3 values; 3 unavailable)
Entities with missing values
rubidium | cesium | francium (total: 3)
Ranked values

| | visual | ratios | 3 | lithium | | 0.66 | 1 2 | sodium | | 0.83 | 1.25 1 | potassium | | 1 | 1.51
Plot
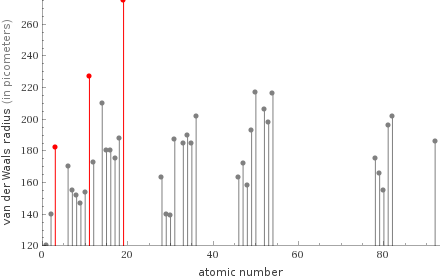
Plot
Periodic table location
Periodic table location
Atomic radii properties
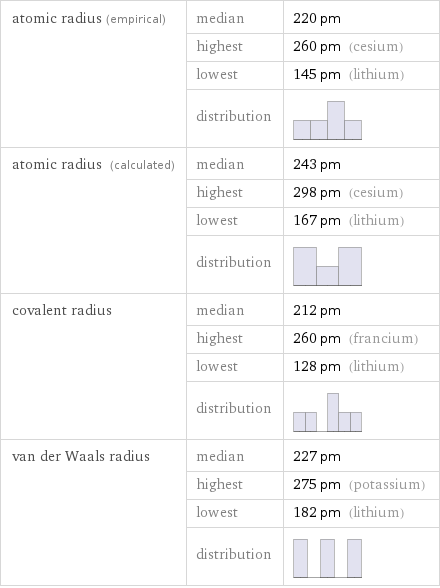
atomic radius (empirical) | median | 220 pm | highest | 260 pm (cesium) | lowest | 145 pm (lithium) | distribution | atomic radius (calculated) | median | 243 pm | highest | 298 pm (cesium) | lowest | 167 pm (lithium) | distribution | covalent radius | median | 212 pm | highest | 260 pm (francium) | lowest | 128 pm (lithium) | distribution | van der Waals radius | median | 227 pm | highest | 275 pm (potassium) | lowest | 182 pm (lithium) | distribution |
Units

Van der Waals radius rankings
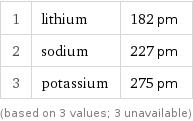
1 | lithium | 182 pm 2 | sodium | 227 pm 3 | potassium | 275 pm (based on 3 values; 3 unavailable)
Unit conversions for median van der Waals radius 227 pm
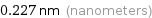
0.227 nm (nanometers)
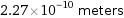
2.27×10^-10 meters
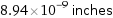
8.94×10^-9 inches
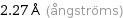
2.27 Å (ångströms)
Comparisons for median van der Waals radius 227 pm
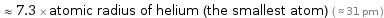
≈ 7.3 × atomic radius of helium (the smallest atom) ( ≈ 31 pm )
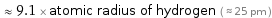
≈ 9.1 × atomic radius of hydrogen ( ≈ 25 pm )