Input interpretation
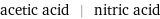
acetic acid | nitric acid
Chemical names and formulas
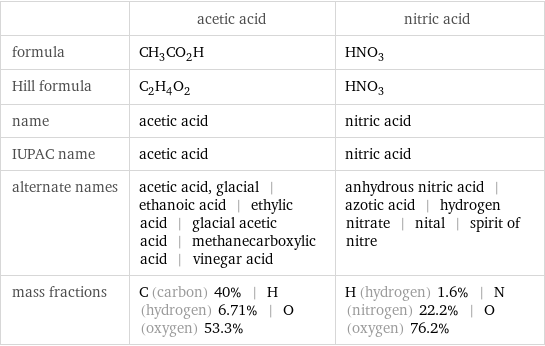
| acetic acid | nitric acid formula | CH_3CO_2H | HNO_3 Hill formula | C_2H_4O_2 | HNO_3 name | acetic acid | nitric acid IUPAC name | acetic acid | nitric acid alternate names | acetic acid, glacial | ethanoic acid | ethylic acid | glacial acetic acid | methanecarboxylic acid | vinegar acid | anhydrous nitric acid | azotic acid | hydrogen nitrate | nital | spirit of nitre mass fractions | C (carbon) 40% | H (hydrogen) 6.71% | O (oxygen) 53.3% | H (hydrogen) 1.6% | N (nitrogen) 22.2% | O (oxygen) 76.2%
Structure diagrams
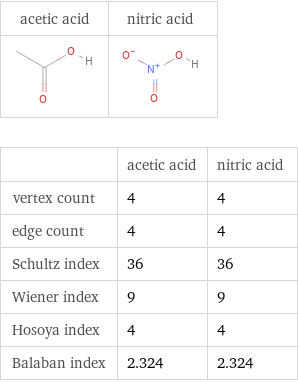
| acetic acid | nitric acid vertex count | 4 | 4 edge count | 4 | 4 Schultz index | 36 | 36 Wiener index | 9 | 9 Hosoya index | 4 | 4 Balaban index | 2.324 | 2.324
3D structure
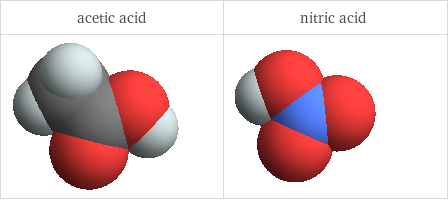
3D structure
Basic properties
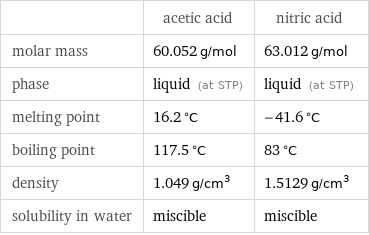
| acetic acid | nitric acid molar mass | 60.052 g/mol | 63.012 g/mol phase | liquid (at STP) | liquid (at STP) melting point | 16.2 °C | -41.6 °C boiling point | 117.5 °C | 83 °C density | 1.049 g/cm^3 | 1.5129 g/cm^3 solubility in water | miscible | miscible
Units

Hydrophobicity and permeability properties

| acetic acid predicted LogP hydrophobicity | -0.12 experimental LogS | 1.22 predicted LogS | 0.73
Liquid properties
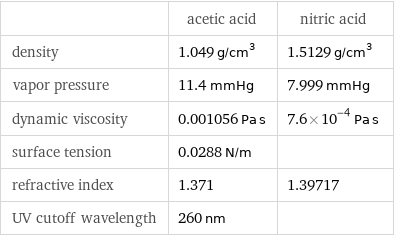
| acetic acid | nitric acid density | 1.049 g/cm^3 | 1.5129 g/cm^3 vapor pressure | 11.4 mmHg | 7.999 mmHg dynamic viscosity | 0.001056 Pa s | 7.6×10^-4 Pa s surface tension | 0.0288 N/m | refractive index | 1.371 | 1.39717 UV cutoff wavelength | 260 nm |
Units

Thermodynamic properties
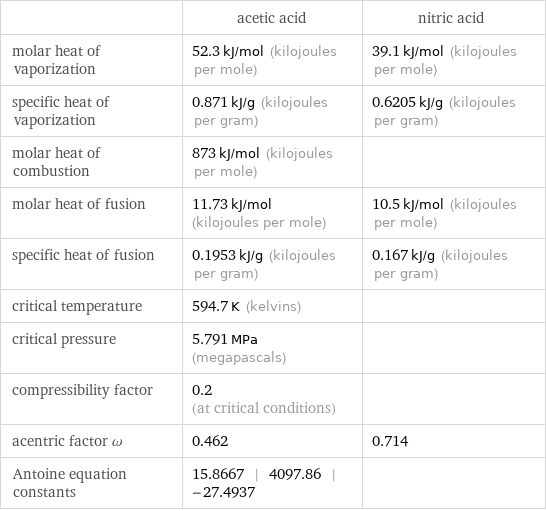
| acetic acid | nitric acid molar heat of vaporization | 52.3 kJ/mol (kilojoules per mole) | 39.1 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.871 kJ/g (kilojoules per gram) | 0.6205 kJ/g (kilojoules per gram) molar heat of combustion | 873 kJ/mol (kilojoules per mole) | molar heat of fusion | 11.73 kJ/mol (kilojoules per mole) | 10.5 kJ/mol (kilojoules per mole) specific heat of fusion | 0.1953 kJ/g (kilojoules per gram) | 0.167 kJ/g (kilojoules per gram) critical temperature | 594.7 K (kelvins) | critical pressure | 5.791 MPa (megapascals) | compressibility factor | 0.2 (at critical conditions) | acentric factor ω | 0.462 | 0.714 Antoine equation constants | 15.8667 | 4097.86 | -27.4937 |
Chemical identifiers
(O)[O-]](../image_source/c3f6145b97be610afe1bdb1624baa91a.png)
| acetic acid | nitric acid CAS number | 64-19-7 | 7697-37-2 Beilstein number | 506007 | PubChem CID number | 176 | 944 PubChem SID number | 3335 | 24853516 SMILES identifier | CC(=O)O | [N+](=O)(O)[O-]
NFPA label

NFPA label