Input interpretation

calcium carbonate
Chemical names and formulas
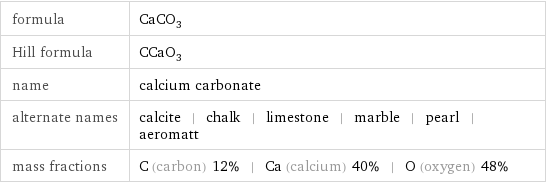
formula | CaCO_3 Hill formula | CCaO_3 name | calcium carbonate alternate names | calcite | chalk | limestone | marble | pearl | aeromatt mass fractions | C (carbon) 12% | Ca (calcium) 40% | O (oxygen) 48%
Structure diagram
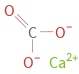
Structure diagram
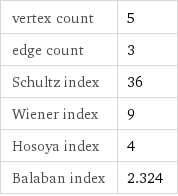
vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties
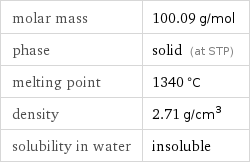
molar mass | 100.09 g/mol phase | solid (at STP) melting point | 1340 °C density | 2.71 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)

density | 2.71 g/cm^3
Units

Thermodynamic properties
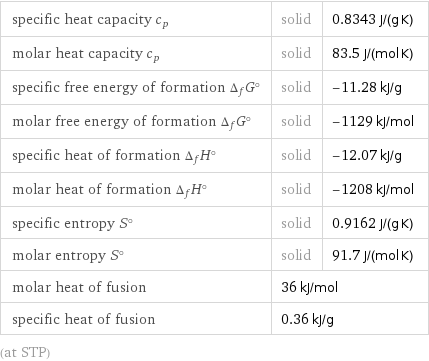
specific heat capacity c_p | solid | 0.8343 J/(g K) molar heat capacity c_p | solid | 83.5 J/(mol K) specific free energy of formation Δ_fG° | solid | -11.28 kJ/g molar free energy of formation Δ_fG° | solid | -1129 kJ/mol specific heat of formation Δ_fH° | solid | -12.07 kJ/g molar heat of formation Δ_fH° | solid | -1208 kJ/mol specific entropy S° | solid | 0.9162 J/(g K) molar entropy S° | solid | 91.7 J/(mol K) molar heat of fusion | 36 kJ/mol | specific heat of fusion | 0.36 kJ/g | (at STP)
Chemical identifiers
![CAS number | 471-34-1 Beilstein number | 8008338 PubChem CID number | 10112 SMILES identifier | C(=O)([O-])[O-].[Ca+2] InChI identifier | InChI=1/CH2O3.Ca/c2-1(3)4;/h(H2, 2, 3, 4);/q;+2/p-2/fCO3.Ca/q-2;m EU number | 215-279-6 Gmelin number | 8544 RTECS number | FF9335000 MDL number | MFCD00010906](../image_source/bb910f3de98447c774235303c4820c6e.png)
CAS number | 471-34-1 Beilstein number | 8008338 PubChem CID number | 10112 SMILES identifier | C(=O)([O-])[O-].[Ca+2] InChI identifier | InChI=1/CH2O3.Ca/c2-1(3)4;/h(H2, 2, 3, 4);/q;+2/p-2/fCO3.Ca/q-2;m EU number | 215-279-6 Gmelin number | 8544 RTECS number | FF9335000 MDL number | MFCD00010906
NFPA label

NFPA label

NFPA health rating | 1 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties
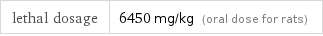
lethal dosage | 6450 mg/kg (oral dose for rats)

probable lethal dose for man | 1 L (liter) RTECS classes | primary irritant
Ion equivalents

Ca^(2+) (calcium cation) | 1 (CO_3)^(2-) (carbonate anion) | 1