Input interpretation

beryllium | specific heat at STP
Result
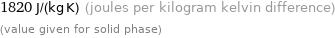
1820 J/(kg K) (joules per kilogram kelvin difference) (value given for solid phase)
Unit conversions
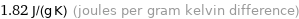
1.82 J/(g K) (joules per gram kelvin difference)
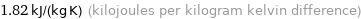
1.82 kJ/(kg K) (kilojoules per kilogram kelvin difference)
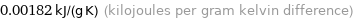
0.00182 kJ/(g K) (kilojoules per gram kelvin difference)
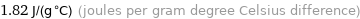
1.82 J/(g °C) (joules per gram degree Celsius difference)
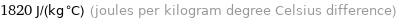
1820 J/(kg °C) (joules per kilogram degree Celsius difference)

0.4347 BTU_IT/(lb °F) (IT British thermal units per pound degree Fahrenheit difference)
Comparisons
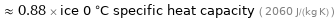
≈ 0.88 × ice 0 °C specific heat capacity ( 2060 J/(kg K) )

≈ 0.91 × oil specific heat capacity ( 2000 J/(kg K) )
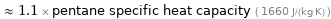
≈ 1.1 × pentane specific heat capacity ( 1660 J/(kg K) )
Thermodynamic properties
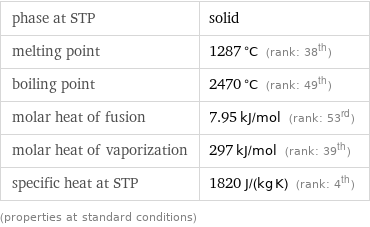
phase at STP | solid melting point | 1287 °C (rank: 38th) boiling point | 2470 °C (rank: 49th) molar heat of fusion | 7.95 kJ/mol (rank: 53rd) molar heat of vaporization | 297 kJ/mol (rank: 39th) specific heat at STP | 1820 J/(kg K) (rank: 4th) (properties at standard conditions)