Input interpretation

group 10 elements | first molar ionization energy
Results
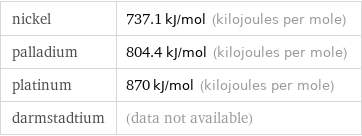
nickel | 737.1 kJ/mol (kilojoules per mole) palladium | 804.4 kJ/mol (kilojoules per mole) platinum | 870 kJ/mol (kilojoules per mole) darmstadtium | (data not available)

group 10 elements | first molar ionization energy | 804.4 kJ/mol (kilojoules per mole) (median) (based on 3 values; 1 unavailable)
Ranked values

| | visual | ratios | 3 | nickel | | 0.847 | 1 2 | palladium | | 0.92 | 1.091 1 | platinum | | 1 | 1.18
Unit conversions for median first molar ionization energy 804.4 kJ/mol

0.8044 MJ/mol (megajoules per mole)
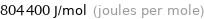
804400 J/mol (joules per mole)
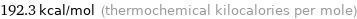
192.3 kcal/mol (thermochemical kilocalories per mole)

8.337 eV (molar electronvolts)
Comparisons for median first molar ionization energy 804.4 kJ/mol

≈ 0.34 × molar energy required to remove the first electron from helium ( 2372 kJ/mol )
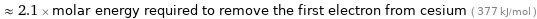
≈ 2.1 × molar energy required to remove the first electron from cesium ( 377 kJ/mol )