Input interpretation
alkaline earth metals | atomic diameter
Summary
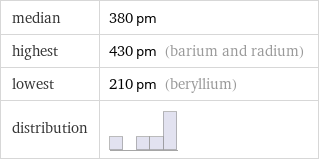
median | 380 pm highest | 430 pm (barium and radium) lowest | 210 pm (beryllium) distribution |
Units

Ranked values
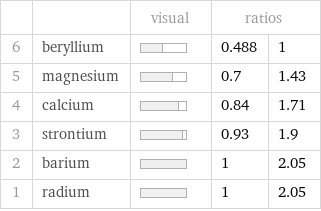
| | visual | ratios | 6 | beryllium | | 0.488 | 1 5 | magnesium | | 0.7 | 1.43 4 | calcium | | 0.84 | 1.71 3 | strontium | | 0.93 | 1.9 2 | barium | | 1 | 2.05 1 | radium | | 1 | 2.05
Plot
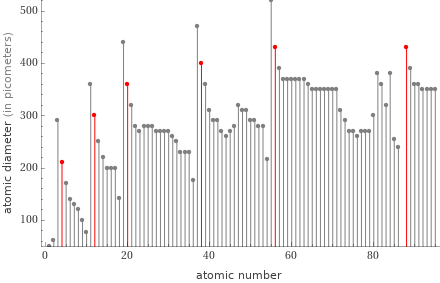
Plot
Periodic table location
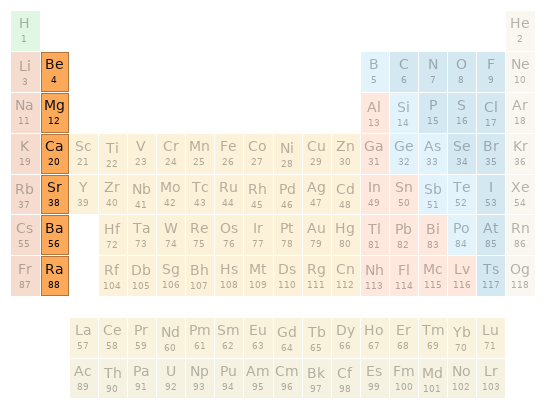
Periodic table location
Atomic radii properties
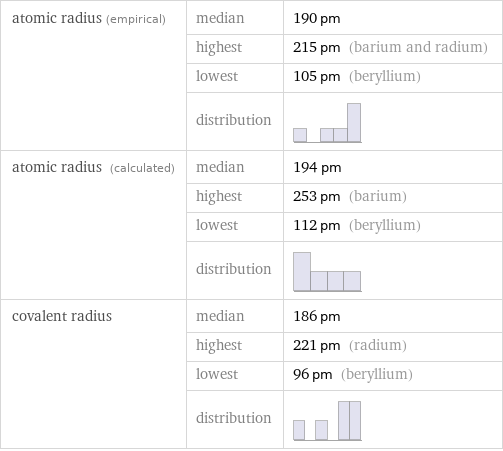
atomic radius (empirical) | median | 190 pm | highest | 215 pm (barium and radium) | lowest | 105 pm (beryllium) | distribution | atomic radius (calculated) | median | 194 pm | highest | 253 pm (barium) | lowest | 112 pm (beryllium) | distribution | covalent radius | median | 186 pm | highest | 221 pm (radium) | lowest | 96 pm (beryllium) | distribution |
Units

Atomic diameter rankings
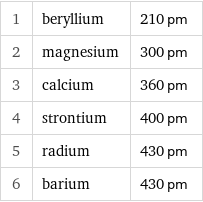
1 | beryllium | 210 pm 2 | magnesium | 300 pm 3 | calcium | 360 pm 4 | strontium | 400 pm 5 | radium | 430 pm 6 | barium | 430 pm
Unit conversions for median atomic diameter 380 pm
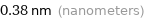
0.38 nm (nanometers)
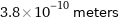
3.8×10^-10 meters
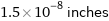
1.5×10^-8 inches
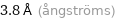
3.8 Å (ångströms)
Comparisons for median atomic diameter 380 pm

≈ 0.4 × carbon nanotube diameter ( ≈ 1 nm )
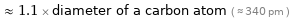
≈ 1.1 × diameter of a carbon atom ( ≈ 340 pm )
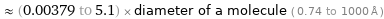
≈ (0.00379 to 5.1) × diameter of a molecule ( 0.74 to 1000 Å )