Input interpretation
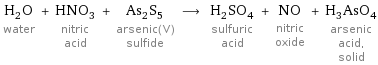
H_2O water + HNO_3 nitric acid + As_2S_5 arsenic(V) sulfide ⟶ H_2SO_4 sulfuric acid + NO nitric oxide + H_3AsO_4 arsenic acid, solid
Balanced equation

Balance the chemical equation algebraically: H_2O + HNO_3 + As_2S_5 ⟶ H_2SO_4 + NO + H_3AsO_4 Add stoichiometric coefficients, c_i, to the reactants and products: c_1 H_2O + c_2 HNO_3 + c_3 As_2S_5 ⟶ c_4 H_2SO_4 + c_5 NO + c_6 H_3AsO_4 Set the number of atoms in the reactants equal to the number of atoms in the products for H, O, N, As and S: H: | 2 c_1 + c_2 = 2 c_4 + 3 c_6 O: | c_1 + 3 c_2 = 4 c_4 + c_5 + 4 c_6 N: | c_2 = c_5 As: | 2 c_3 = c_6 S: | 5 c_3 = c_4 Since the coefficients are relative quantities and underdetermined, choose a coefficient to set arbitrarily. To keep the coefficients small, the arbitrary value is ordinarily one. For instance, set c_3 = 1 and solve the system of equations for the remaining coefficients: c_1 = 4/3 c_2 = 40/3 c_3 = 1 c_4 = 5 c_5 = 40/3 c_6 = 2 Multiply by the least common denominator, 3, to eliminate fractional coefficients: c_1 = 4 c_2 = 40 c_3 = 3 c_4 = 15 c_5 = 40 c_6 = 6 Substitute the coefficients into the chemical reaction to obtain the balanced equation: Answer: | | 4 H_2O + 40 HNO_3 + 3 As_2S_5 ⟶ 15 H_2SO_4 + 40 NO + 6 H_3AsO_4
Structures
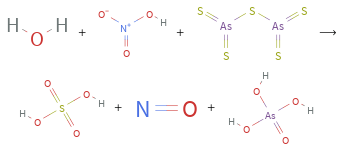
+ + ⟶ + +
Names
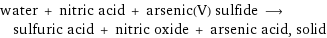
water + nitric acid + arsenic(V) sulfide ⟶ sulfuric acid + nitric oxide + arsenic acid, solid
Equilibrium constant
![Construct the equilibrium constant, K, expression for: H_2O + HNO_3 + As_2S_5 ⟶ H_2SO_4 + NO + H_3AsO_4 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the activity expression for each chemical species. • Use the activity expressions to build the equilibrium constant expression. Write the balanced chemical equation: 4 H_2O + 40 HNO_3 + 3 As_2S_5 ⟶ 15 H_2SO_4 + 40 NO + 6 H_3AsO_4 Assign stoichiometric numbers, ν_i, using the stoichiometric coefficients, c_i, from the balanced chemical equation in the following manner: ν_i = -c_i for reactants and ν_i = c_i for products: chemical species | c_i | ν_i H_2O | 4 | -4 HNO_3 | 40 | -40 As_2S_5 | 3 | -3 H_2SO_4 | 15 | 15 NO | 40 | 40 H_3AsO_4 | 6 | 6 Assemble the activity expressions accounting for the state of matter and ν_i: chemical species | c_i | ν_i | activity expression H_2O | 4 | -4 | ([H2O])^(-4) HNO_3 | 40 | -40 | ([HNO3])^(-40) As_2S_5 | 3 | -3 | ([As2S5])^(-3) H_2SO_4 | 15 | 15 | ([H2SO4])^15 NO | 40 | 40 | ([NO])^40 H_3AsO_4 | 6 | 6 | ([H3AsO4])^6 The equilibrium constant symbol in the concentration basis is: K_c Mulitply the activity expressions to arrive at the K_c expression: Answer: | | K_c = ([H2O])^(-4) ([HNO3])^(-40) ([As2S5])^(-3) ([H2SO4])^15 ([NO])^40 ([H3AsO4])^6 = (([H2SO4])^15 ([NO])^40 ([H3AsO4])^6)/(([H2O])^4 ([HNO3])^40 ([As2S5])^3)](../image_source/1f9cdc25fa5928547a31d072d6bd471f.png)
Construct the equilibrium constant, K, expression for: H_2O + HNO_3 + As_2S_5 ⟶ H_2SO_4 + NO + H_3AsO_4 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the activity expression for each chemical species. • Use the activity expressions to build the equilibrium constant expression. Write the balanced chemical equation: 4 H_2O + 40 HNO_3 + 3 As_2S_5 ⟶ 15 H_2SO_4 + 40 NO + 6 H_3AsO_4 Assign stoichiometric numbers, ν_i, using the stoichiometric coefficients, c_i, from the balanced chemical equation in the following manner: ν_i = -c_i for reactants and ν_i = c_i for products: chemical species | c_i | ν_i H_2O | 4 | -4 HNO_3 | 40 | -40 As_2S_5 | 3 | -3 H_2SO_4 | 15 | 15 NO | 40 | 40 H_3AsO_4 | 6 | 6 Assemble the activity expressions accounting for the state of matter and ν_i: chemical species | c_i | ν_i | activity expression H_2O | 4 | -4 | ([H2O])^(-4) HNO_3 | 40 | -40 | ([HNO3])^(-40) As_2S_5 | 3 | -3 | ([As2S5])^(-3) H_2SO_4 | 15 | 15 | ([H2SO4])^15 NO | 40 | 40 | ([NO])^40 H_3AsO_4 | 6 | 6 | ([H3AsO4])^6 The equilibrium constant symbol in the concentration basis is: K_c Mulitply the activity expressions to arrive at the K_c expression: Answer: | | K_c = ([H2O])^(-4) ([HNO3])^(-40) ([As2S5])^(-3) ([H2SO4])^15 ([NO])^40 ([H3AsO4])^6 = (([H2SO4])^15 ([NO])^40 ([H3AsO4])^6)/(([H2O])^4 ([HNO3])^40 ([As2S5])^3)
Rate of reaction
![Construct the rate of reaction expression for: H_2O + HNO_3 + As_2S_5 ⟶ H_2SO_4 + NO + H_3AsO_4 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the rate term for each chemical species. • Write the rate of reaction expression. Write the balanced chemical equation: 4 H_2O + 40 HNO_3 + 3 As_2S_5 ⟶ 15 H_2SO_4 + 40 NO + 6 H_3AsO_4 Assign stoichiometric numbers, ν_i, using the stoichiometric coefficients, c_i, from the balanced chemical equation in the following manner: ν_i = -c_i for reactants and ν_i = c_i for products: chemical species | c_i | ν_i H_2O | 4 | -4 HNO_3 | 40 | -40 As_2S_5 | 3 | -3 H_2SO_4 | 15 | 15 NO | 40 | 40 H_3AsO_4 | 6 | 6 The rate term for each chemical species, B_i, is 1/ν_i(Δ[B_i])/(Δt) where [B_i] is the amount concentration and t is time: chemical species | c_i | ν_i | rate term H_2O | 4 | -4 | -1/4 (Δ[H2O])/(Δt) HNO_3 | 40 | -40 | -1/40 (Δ[HNO3])/(Δt) As_2S_5 | 3 | -3 | -1/3 (Δ[As2S5])/(Δt) H_2SO_4 | 15 | 15 | 1/15 (Δ[H2SO4])/(Δt) NO | 40 | 40 | 1/40 (Δ[NO])/(Δt) H_3AsO_4 | 6 | 6 | 1/6 (Δ[H3AsO4])/(Δt) (for infinitesimal rate of change, replace Δ with d) Set the rate terms equal to each other to arrive at the rate expression: Answer: | | rate = -1/4 (Δ[H2O])/(Δt) = -1/40 (Δ[HNO3])/(Δt) = -1/3 (Δ[As2S5])/(Δt) = 1/15 (Δ[H2SO4])/(Δt) = 1/40 (Δ[NO])/(Δt) = 1/6 (Δ[H3AsO4])/(Δt) (assuming constant volume and no accumulation of intermediates or side products)](../image_source/310176f604425b55fb464e96283f4533.png)
Construct the rate of reaction expression for: H_2O + HNO_3 + As_2S_5 ⟶ H_2SO_4 + NO + H_3AsO_4 Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the rate term for each chemical species. • Write the rate of reaction expression. Write the balanced chemical equation: 4 H_2O + 40 HNO_3 + 3 As_2S_5 ⟶ 15 H_2SO_4 + 40 NO + 6 H_3AsO_4 Assign stoichiometric numbers, ν_i, using the stoichiometric coefficients, c_i, from the balanced chemical equation in the following manner: ν_i = -c_i for reactants and ν_i = c_i for products: chemical species | c_i | ν_i H_2O | 4 | -4 HNO_3 | 40 | -40 As_2S_5 | 3 | -3 H_2SO_4 | 15 | 15 NO | 40 | 40 H_3AsO_4 | 6 | 6 The rate term for each chemical species, B_i, is 1/ν_i(Δ[B_i])/(Δt) where [B_i] is the amount concentration and t is time: chemical species | c_i | ν_i | rate term H_2O | 4 | -4 | -1/4 (Δ[H2O])/(Δt) HNO_3 | 40 | -40 | -1/40 (Δ[HNO3])/(Δt) As_2S_5 | 3 | -3 | -1/3 (Δ[As2S5])/(Δt) H_2SO_4 | 15 | 15 | 1/15 (Δ[H2SO4])/(Δt) NO | 40 | 40 | 1/40 (Δ[NO])/(Δt) H_3AsO_4 | 6 | 6 | 1/6 (Δ[H3AsO4])/(Δt) (for infinitesimal rate of change, replace Δ with d) Set the rate terms equal to each other to arrive at the rate expression: Answer: | | rate = -1/4 (Δ[H2O])/(Δt) = -1/40 (Δ[HNO3])/(Δt) = -1/3 (Δ[As2S5])/(Δt) = 1/15 (Δ[H2SO4])/(Δt) = 1/40 (Δ[NO])/(Δt) = 1/6 (Δ[H3AsO4])/(Δt) (assuming constant volume and no accumulation of intermediates or side products)
Chemical names and formulas
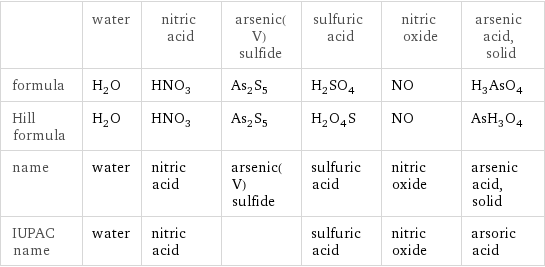
| water | nitric acid | arsenic(V) sulfide | sulfuric acid | nitric oxide | arsenic acid, solid formula | H_2O | HNO_3 | As_2S_5 | H_2SO_4 | NO | H_3AsO_4 Hill formula | H_2O | HNO_3 | As_2S_5 | H_2O_4S | NO | AsH_3O_4 name | water | nitric acid | arsenic(V) sulfide | sulfuric acid | nitric oxide | arsenic acid, solid IUPAC name | water | nitric acid | | sulfuric acid | nitric oxide | arsoric acid